Heme and lithospermic acid synergistically inhibit aggregation of human islet amyloid polypeptide: A novel hIAPP inhibitor for the potential therapy of type 2 diabetes
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Amyloid deposition of human islet amyloid polypeptide (hIAPP) is closely linked to the pathogenesis and progression of type 2 diabetes mellitus (T2DM). Developing effective inhibitors to suppress hIAPP aggregation holds significant therapeutic potential for the prevention and treatment of T2DM. Recent researches indicate that both heme and lithospermic acid (LPA) can inhibit hIAPP aggregation. However, heme is prone to induce protein damage under oxidative stress, while LPA exhibits limited inhibitory efficacy despite its antioxidant properties. To overcome these limitations, we aimed to develop a dual-component inhibitor comprising heme and LPA. thioflavin T (ThT) fluorescence, transmission electron microscopy (TEM), circular dichroism (CD) and gel electrophoresis were combined to observe the inhibitory efficacy of heme-LPA co-formulation on hIAPP aggregation. The results demonstrate that LPA and heme can synergistically inhibit hIAPP aggregation. The inhibitory effect of heme-LPA co-formulation on hIAPP aggregation is significantly stronger than that of either component alone. The heme-LPA not only prevents the complete conversion of hIAPP into β-sheet fibrillar structures but also maintains its active monomeric conformation for extended periods. Furthermore, peroxidase activity assays revealed that the presence of LPA significantly reduces the peroxidase activity of heme in a concentration-dependent manner and attenuates peptide nitration damage under H₂O₂-NO₂− oxidative stress. At a heme-to-LPA ratio of 1:4, peptide nitration bands were virtually undetectable. These findings indicate that the dual-component inhibitor heme-LPA represents an efficient and safe strategy for inhibiting hIAPP aggregation. This research provides an important avenue for developing novel anti-hIAPP aggregation inhibitors for the prevention and treatment of T2DM.
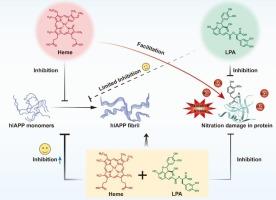
血红素和石精酸协同抑制人胰岛淀粉样多肽聚集:一种新的hIAPP抑制剂,有望治疗2型糖尿病。
人胰岛淀粉样多肽(hIAPP)的淀粉样沉积与2型糖尿病(T2DM)的发病和进展密切相关。开发有效的抑制hIAPP聚集的抑制剂对预防和治疗T2DM具有重要的治疗潜力。最近的研究表明,血红素和石精酸(LPA)都能抑制hIAPP的聚集。然而,血红素在氧化应激下容易诱导蛋白质损伤,而LPA虽然具有抗氧化特性,但其抑制作用有限。为了克服这些限制,我们旨在开发一种含有血红素和LPA的双组分抑制剂。结合硫黄素T (ThT)荧光、透射电镜(TEM)、圆二色性(CD)和凝胶电泳观察血红素- lpa共配物对hIAPP聚集的抑制作用。结果表明,LPA和血红素可以协同抑制hIAPP的聚集。血红素- lpa共制剂对hIAPP聚集的抑制作用明显强于单独使用任何一种成分。血红素- lpa不仅可以阻止hIAPP完全转化为β-片状纤维结构,还可以延长其活性单体构象。此外,过氧化物酶活性测定表明,LPA的存在显著降低血红素过氧化物酶活性,并以浓度依赖的方式减弱H₂O₂- no₂氧化应激下肽的硝化损伤。血红素与lpa的比例为1:4时,几乎无法检测到肽硝化带。这些发现表明,双组分抑制剂血红素- lpa是一种有效且安全的抑制hIAPP聚集的策略。本研究为开发新型抗hiapp聚集抑制剂预防和治疗T2DM提供了重要途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


