Mechanism of synergistic enhancement of α-secretase (ADAM10) activity by EGCG and FA
引用次数: 0
Abstract
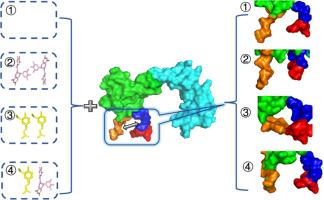
Enhancing the activity of α-secretase has emerged as a potential therapeutic strategy for treating Alzheimer's disease (AD). The exploration of small molecules that can enhance α-secretase activity and their mechanisms provides insights for future AD treatments and the development of novel activators. In this study, ADAM10, a major α-secretase, is used as a model and is bound with the ligands (−)-epigallocatechin-3-gallate (EGCG) and ferulic acid (FA) in a 1:2 ratio (ADAM10:EGCG/FA = 1:2/2) and equimolar ratio (ADAM10:EGCG:FA = 1:1:1) to investigate the effects on ADAM10 activation and reveal the synergistic mechanism of the EGCG and FA combination. The activity of ADAM10 was enhanced by the combination of EGCG and FA, compared to that achieved with EGCG or FA alone, where EGCG plays a dominant role, whereas FA plays a supportive role. The combined use of EGCG induces strong hydrophobic interactions between ADAM10 and FA, causing FA to dissociate from the S1 domain, thereby preventing the inhibition of ADAM10 activity by pure FA. The presence of FA allows EGCG to bind more precisely within the active cavity of ADAM10, thereby increasing the binding strength. Overall, the combination of EGCG and FA significantly increased the distance between the S1 domain and the cysteine-rich C-terminus, further opening up the cavity containing the active sites, consequently exposing more active sites and enhancing the activity of ADAM10.
EGCG和FA协同增强α-分泌酶(ADAM10)活性的机制
增强α-分泌酶活性已成为治疗阿尔茨海默病(AD)的潜在治疗策略。探索能够增强α-分泌酶活性的小分子及其机制,为未来AD的治疗和新型激活剂的开发提供了新的思路。本研究以α-分泌酶ADAM10为模型,以1:2的比例(ADAM10:EGCG/FA = 1:2/2)和等摩尔比例(ADAM10:EGCG:FA = 1:1:1)与表没食子儿茶素-3-没食子酸酯(EGCG -3-没食子酸酯)和阿魏酸(FA)结合,研究其对ADAM10活化的影响,揭示EGCG和FA联合的协同作用机制。与单独使用EGCG或FA相比,EGCG和FA联合使用可以增强ADAM10的活性,其中EGCG起主导作用,而FA起支持作用。EGCG的联合使用诱导了ADAM10和FA之间强烈的疏水相互作用,导致FA从S1结构域解离,从而防止了纯FA对ADAM10活性的抑制。FA的存在允许EGCG更精确地结合在ADAM10的活性腔内,从而增加了结合强度。综上所述,EGCG和FA的结合显著增加了S1结构域与富含半胱氨酸的c端之间的距离,进一步打开了含有活性位点的空腔,从而暴露了更多的活性位点,增强了ADAM10的活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


