Tailoring memory performance via engineering conjugated bridges in benzo[c][1,2,5]thiadiazole based donor–acceptor small molecules
引用次数: 0
Abstract
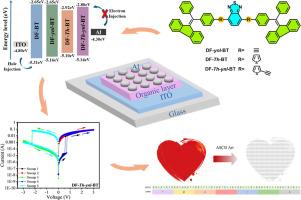
Tuning the conjugated bridges between the electron-donor and electron-acceptor moieties plays a crucial role in enhancing the memristive properties of organic materials, yet it is rarely reported. Herein, we designed and synthesized four donor–acceptor (D-A) organic small molecules, namely 4,7-bis(4-((9H-fluoren-9-ylidene)(phenyl)methyl)phenyl)benzo[c][1,2,5]thiadiazole (DF-BT), 4,7-bis((4-((9H-fluoren-9-ylidene)(phenyl)methyl)phenyl)ethynyl)benzo[c][1,2,5]thiadiazole (DF-ynl-BT), 4,7-bis(5-(4-((9H-fluoren-9-ylidene)(phenyl)methyl)phenyl)thiophen-2-yl)benzo[c][1,2,5]thiadiazole (DF-Th-BT), and 4,7-bis((5-(4-((9H-fluoren-9-ylidene)(phenyl)methyl)phenyl)thiophen-2-yl)ethynyl)benzo[c][1,2,5]thiadiazole (DF-Th-ynl-BT), featuring unique conjugated bridges. These molecules were employed as active layers in resistive random-access memory (RRAM) devices to systematically investigate the influence of conjugation bridges on the electrical parameters. The results revealed that devices based on DF-BT, DF-ynl-BT, and DF-Th-BT exhibited write-once-read-many-times (WORM) characteristics, while the DF-Th-ynl-BT-based device demonstrated stable Flash-type switching behavior. Compared to DF-BT, memory devices utilizing DF-ynl-BT, DF-Th-BT, and DF-Th-ynl-BT, which incorporate additional conjugated bridges, exhibited nonvolatile memory properties with reduced threshold voltages, an improved ON/OFF current ratio, enhanced stability, and better uniformity. These findings demonstrated that tailoring the conjugated bridges in D-A molecules can effectively modulate resistive memory behavior and enhance device performance. Furthermore, the DF-Th-ynl-BT-based device was successfully integrated into logic gate circuits and display functions, highlighting its significant potential for applications in artificial intelligence (AI) neural networks.
基于工程共轭桥的苯并[c][1,2,5]噻二唑小分子给受体修饰记忆性能
调整电子给体和电子受体之间的共轭桥在提高有机材料的记忆电阻性能中起着至关重要的作用,但很少报道。本文设计并合成了4种给受体(D-A)有机小分子,即4,7-二(4-(9h -芴-9-基)(苯基)甲基)苯基)苯并[c][1,2,5]噻二唑(DF-BT), 4,7-二((4-((9h -芴-9-基)(苯基)甲基)苯基)乙基)苯并[c][1,2,5]噻二唑(DF-ynl-BT), 4,7-二(5-(4-((9h -芴-9-基)(苯基)甲基)苯基)噻吩-2-基)苯并[c][1,2,5]噻二唑(DF-Th-BT),和4,7-二((5-(4-((9h -芴-9-基)(苯基)甲基)苯基)噻吩-2-基)乙基)苯并[c][1,2,5]噻二唑(DF-Th-ynl-BT),具有独特的共轭桥。将这些分子作为有源层应用于电阻式随机存取存储器(RRAM)器件中,系统地研究了共轭桥对电参数的影响。结果表明,基于DF-BT、DF-ynl-BT和DF-Th-BT的器件表现出写一次读多次(WORM)特性,而基于df - th -ynl- bt的器件表现出稳定的flash型开关行为。与DF-BT相比,采用DF-ynl-BT、DF-Th-BT和DF-Th-ynl-BT的存储器件采用了额外的共轭桥,具有降低阈值电压、提高开/关电流比、增强稳定性和更好的均匀性等非易失性存储特性。这些发现表明,调整D-A分子中的共轭桥可以有效地调节电阻性记忆行为并提高器件性能。此外,基于df - th -ynl- bt的器件已成功集成到逻辑门电路和显示功能中,突出了其在人工智能(AI)神经网络中的应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


