Biological and molecular characterisation of in vitro selected miltefosine-resistant Leishmania amazonensis lines
IF 3.4
2区 医学
Q1 PARASITOLOGY
International Journal for Parasitology: Drugs and Drug Resistance
Pub Date : 2025-09-26
DOI:10.1016/j.ijpddr.2025.100617
引用次数: 0
Abstract
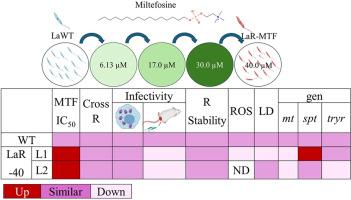
Miltefosine (MTF) is currently the only available oral treatment for leishmaniasis. However, increasing reports of therapeutic failure have raised concerns about emerging resistance. This study aimed to investigate the effects of reduced MTF susceptibility loss in the protozoan parasite Leishmania (Leishmania) amazonensis, with a particular focus on its impact on key biological and molecular parameters. Two distinct Leishmania lines (LaR-40 Line 1 and Line 2) were generated through stepwise in vitro selection with increasing concentrations of MTF, reaching up to 40 μM MTF. They were compared to their wild-type counterpart (LaWT). After 12 weeks of selection, LaR-40 promastigotes exhibited IC50 values that were 4- to 8-fold higher than those of LaWT, with resistance remaining stable even after three months without drug pressure and following passage through BALB/c mice. No cross-resistance was detected against pentamidine, ketoconazole, or amphotericin B. MTF-resistant parasites exhibited reduced reactive oxygen species production, reduced lipid droplets (LD) abundance (in LaR-40 Line 1), delayed lesion onset, and smaller cutaneous lesions in mice, while maintaining normal infectivity in THP-1 macrophages. Quantitative RT-PCR analysis revealed consistent downregulation of the miltefosine transporter (mt) gene in both MTF-resistant lines, indicating that reduced drug uptake is the main mechanism underlying resistance. Line-specific changes, such as the upregulation of the serine palmitoyltransferase (spt) gene or the downregulation of the trypanothione reductase (tryr) gene, suggest that distinct metabolic pathways may act in a compensatory manner to reinforce resistance once transporter function is impaired. These findings indicate that MTF resistance in L. amazonensis is polygenic, stable, and adaptable. Routine monitoring of mt gene expression, combined with therapeutic strategies that target lipid or redox metabolism alongside drug uptake pathways, may help preserve the efficacy of current treatment regimens against leishmaniasis.
亚马逊利什曼原虫耐米特氟辛体外菌株的生物学和分子特性分析。
米替福辛(MTF)是目前唯一可用于治疗利什曼病的口服药物。然而,越来越多的治疗失败报告引起了人们对新出现的耐药性的关注。本研究旨在探讨MTF敏感性降低对原生动物亚马逊利什曼原虫(Leishmania)的影响,特别关注其对关键生物学和分子参数的影响。随着MTF浓度的增加,逐步筛选得到两个不同的利什曼原虫系(LaR-40 Line 1和Line 2), MTF浓度达到40 μM。将它们与野生型对应物(LaWT)进行比较。选择12周后,LaR-40 promastigotes的IC50值比LaWT高4- 8倍,即使在没有药物压力的3个月后并通过BALB/c小鼠,其抗性仍保持稳定。未检测到对喷他脒、酮康唑或两性霉素b的交叉耐药。mtf耐药的寄生虫表现出活性氧产生减少,脂滴(LD)丰度降低(在LaR-40 Line 1中),病变发作延迟,小鼠皮肤病变变小,同时保持THP-1巨噬细胞的正常感染性。定量RT-PCR分析显示,在两种mtf耐药品系中,米替氟转运蛋白(mt)基因一致下调,表明药物摄取减少是耐药的主要机制。系特异性变化,如丝氨酸棕榈酰转移酶(spt)基因的上调或trypanothione还原酶(tryr)基因的下调,表明一旦转运体功能受损,不同的代谢途径可能以代偿方式增强抗性。这些结果表明,亚马孙猕猴桃对MTF的抗性具有多基因性、稳定性和适应性。常规监测mt基因表达,结合针对脂质或氧化还原代谢以及药物摄取途径的治疗策略,可能有助于保持当前治疗方案对利什曼病的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal for Parasitology: Drugs and Drug Resistance
PARASITOLOGY-PHARMACOLOGY & PHARMACY
CiteScore
7.90
自引率
7.50%
发文量
31
审稿时长
48 days
期刊介绍:
The International Journal for Parasitology – Drugs and Drug Resistance is one of a series of specialist, open access journals launched by the International Journal for Parasitology. It publishes the results of original research in the area of anti-parasite drug identification, development and evaluation, and parasite drug resistance. The journal also covers research into natural products as anti-parasitic agents, and bioactive parasite products. Studies can be aimed at unicellular or multicellular parasites of human or veterinary importance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


