Realizing large birefringence via S-substitution and anisotropic arrangement optimization
IF 6.4
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
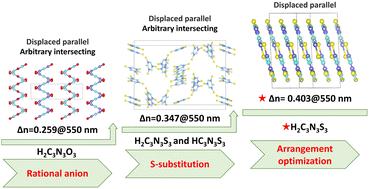
Despite the widespread use of birefringence crystals in optical instruments, the birefringence of commercially available crystals is generally limited (Δn < 0.3), making them less suitable for demanding optical requirements. In this work, three novel birefringent crystals, namely (C2N5H8)(H2C3N3O3)·H2O (1), (C2N5H8)3(H2C3N3S3)(HC3N3S3)·H2O (2), and (C2N5H8)(H2C3N3S3)·H2O (3), were successfully synthesized via a two-step strategy. In their anionic structures, a displaced parallel arrangement is observed among three crystals, combined with a partially distinct arbitrary intersecting arrangement. Further research findings indicate that sulfur (S) substitution (from 1 to 2) and the optimized arrangement of functional groups (from 2 to 3) lead to a significant enhancement in the birefringence. The experimental birefringence values at 550 nm increased from 0.259 (1) to 0.347 (2), reaching 0.403 (3). Remarkably, the birefringence performance of compound 3 ranks third among all reported [C3N3S3]-based materials. Theoretical calculations reveal that its high birefringence is primarily attributed to S-substitution and the optimized arrangement of functional groups. This work provides critical guidance for further exploration of the impact of structure on birefringence performance and opens up new research directions for designing high-performance optical materials.
通过s置换和各向异性排列优化实现大双折射
尽管双折射晶体在光学仪器中广泛使用,但市售晶体的双折射通常是有限的(Δn < 0.3),这使得它们不太适合苛刻的光学要求。本文采用两步法成功合成了(C2N5H8)(H2C3N3O3)·H2O(1)、(C2N5H8)3(H2C3N3S3)(HC3N3S3)·H2O(2)和(C2N5H8)(H2C3N3S3)·H2O(3)三种新型双折射晶体。在它们的阴离子结构中,在三个晶体之间观察到移位的平行排列,并结合了部分不同的任意交叉排列。进一步的研究发现,硫(S)取代(从1到2)和优化的官能团排列(从2到3)导致双折射显著增强。实验双折射值在550 nm处从0.259(1)增加到0.347(2),达到0.403(3)。值得注意的是,化合物3的双折射性能在所有已报道的[C3N3S3]基材料中排名第三。理论计算表明,其高双折射性主要归因于s取代和官能团的优化排列。这项工作为进一步探索结构对双折射性能的影响提供了重要的指导,为高性能光学材料的设计开辟了新的研究方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Frontiers
CHEMISTRY, INORGANIC & NUCLEAR-
CiteScore
10.40
自引率
7.10%
发文量
587
审稿时长
1.2 months
期刊介绍:
The international, high quality journal for interdisciplinary research between inorganic chemistry and related subjects
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


