Frustrated Lewis Pair-Mediated Catalyst Efficiency in ROMP: Mechanistic Insights from Boronic Ester-Functionalized Monomers
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
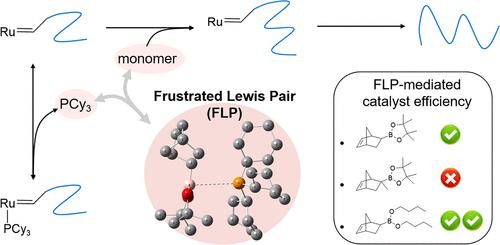
The ring-opening metathesis polymerization (ROMP) of boronic ester-functionalized monomers remains underexplored compared with other polymerization strategies, despite the unique physical and chemical properties offered by boronic esters. Herein, we report an unrecognized mechanistic insight into which boronic ester-functionalized monomers modulate ROMP kinetics via frustrated Lewis pair (FLP) interactions. In situ nuclear magnetic resonance (NMR) spectroscopy and density functional theory (DFT) calculations reveal that the boron atoms in norbornylboronic acid pinacol ester (NB-Bpin) and norbornylboronic acid dibutyl ester (NB-B(OBu)2 ) can associate with tricyclohexylphosphine (PCy3) ligands of Grubbs’ catalyst, forming FLPs that shift the ligand-catalyst binding equilibrium toward active ruthenium species. This FLP-mediated mechanism increases the polymerization rate proportionally to monomer concentration, with NB-B(OBu)2 (flexible butoxy groups) exhibiting a 3.75-fold enhancement compared with a 1.26-fold increase for NB-Bpin (rigid five-membered pinacol boronate). In contrast, methylnorbornylboronic acid pinacol ester (MNB-Bpin), designed with a methyl group adjacent to the boron atom to suppress FLP formation, shows no enhancement in the polymerization rate. Crucially, the weak interaction nature of FLP allows PCy3 to dissociate completely from the polymer product, preserving the material’s intrinsic properties. The resulting boronic ester-functionalized polymer exhibits high thermal stability, optical transparency (>91% ), and dynamic covalent cross-linking characteristics. By elucidating the monomer-ligand interactions in ROMP, this study demonstrates the potential of these FLPs in modulating the catalyst efficiency, and offers broad implications for diverse reaction systems.
在ROMP中受挫的Lewis对介导的催化剂效率:来自硼酯功能化单体的机理见解
尽管硼酯具有独特的物理和化学性质,但与其他聚合策略相比,硼酯功能化单体的开环复分解聚合(ROMP)仍未得到充分的研究。在此,我们报告了一个未被认识的机制洞察,其中硼酯功能化单体通过受挫的路易斯对(FLP)相互作用调节ROMP动力学。原位核磁共振(NMR)和密度泛函理论(DFT)计算表明,降冰片硼酸松醇酯(NB-Bpin)和降冰片硼酸二丁基酯(NB-B(OBu)2)中的硼原子可以与Grubbs催化剂的三环己基膦(PCy3)配体结合,形成FLPs,使配体-催化剂的结合平衡向活性钌态转移。这种由flp介导的机制与单体浓度成比例地提高了聚合速率,NB-B(OBu)2(柔性丁氧基)的聚合速率提高了3.75倍,而NB-Bpin(刚性五元硼酸苄啶)的聚合速率提高了1.26倍。相比之下,甲基降冰片酰基硼酸蒎醇酯(MNB-Bpin)在硼原子附近设计了一个甲基来抑制FLP的形成,但没有提高聚合速度。至关重要的是,FLP的弱相互作用性质允许PCy3与聚合物产品完全分离,保持材料的固有特性。所得的硼酯功能化聚合物具有高热稳定性,光学透明度(>91%)和动态共价交联特性。通过阐明ROMP中单体与配体的相互作用,本研究证明了这些FLPs在调节催化剂效率方面的潜力,并为不同的反应体系提供了广泛的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


