Bis(sulfone) as Bifunctional Reagent for the Alkylarylation of Alkenes and Alkynes under Photoredox Conditions
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we report a strategy for the alkylarylation of unsaturated carbon–carbon bonds, wherein bis(sulfones) serve as dual-source donors to deliver alkyl and aryl groups in a single step, redefining their reactivity paradigm. Both alkenes and alkynes undergo efficient difunctionalization with broad functional group tolerance. Mechanistic studies reveal a cascade involving deprotonation, single-electron oxidation, radical addition and Smiles rearrangement.
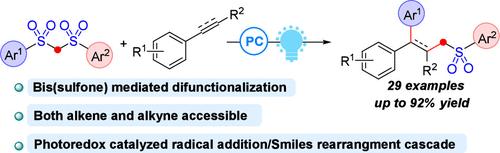
双砜作为光氧化还原条件下烯烃和炔烃烷基化反应的双功能试剂。
在此,我们报告了一种不饱和碳-碳键的烷基化策略,其中双砜作为双源供体,在一个步骤中传递烷基和芳基,重新定义了它们的反应性范式。烯烃和炔都能进行有效的双官能团化,具有广泛的官能团耐受性。机理研究揭示了一个涉及去质子化、单电子氧化、自由基加成和斯迈尔斯重排的级联反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


