OXTR overexpression induces polycystic ovary syndrome-like phenotype via prolactin/p-STAT3 signaling in mice
IF 3.6
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Polycystic ovary syndrome (PCOS), a prevalent endocrine disorder, represents the most common cause of anovulatory infertility. While the oxytocin receptor (OXTR) is well-characterized in parturition and lactation, its role in follicular development remains undefined. In this study, we establish that global OXTR overexpression in female mice (++Oxtr) recapitulates cardinal PCOS features, including hyperandrogenism, oligo-ovulation, and polycystic ovarian changes. ++Oxtr females exhibited distinct ovarian pathology marked by follicular atresia, cystic changes, hemorrhage, and deficient corpus luteum formation. These morphological alterations coincided with profound endocrine dysregulation, featuring hyperprolactinemia, suppressed luteinizing hormone (LH) secretion, and progesterone (P) deficiency, contrasting with preserved fertility in ++Oxtr males. Mechanistically, we identified an OXTR-prolactin (PRL)-p-STAT3 axis as central to PCOS pathogenesis. Corresponding to hyperprolactinemia, persistent activation of nuclear p-STAT3 (Tyr705) in ++Oxtr ovaries - absent in WT controls at pregnancy - upregulated folliculogenesis genes (Lhcgr, Pgr, Leptin, Cyp17a1) while impairing ovulation. Therapeutic intervention with bromocriptine normalized prolactin and progesterone levels, partially restoring ovarian function. Notably, ++Oxtr females developed metabolic dysfunction characterized by insulin resistance and gonadal adiposity despite maintaining lean phenotypes. Our findings position OXTR as a novel upstream regulator of PCOS pathogenesis with hyperprolactinemia, suggesting bromocriptine may have therapeutic value in hyperprolactinemic PCOS cases. These insights open new avenues for targeted PCOS interventions.
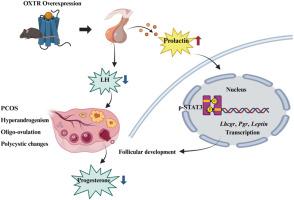
OXTR过表达通过催乳素/p-STAT3信号诱导小鼠多囊卵巢综合征样表型
多囊卵巢综合征(PCOS)是一种常见的内分泌紊乱,是无排卵性不孕的最常见原因。虽然催产素受体(OXTR)在分娩和哺乳中有很好的特征,但其在卵泡发育中的作用仍不清楚。在这项研究中,我们证实雌性小鼠中OXTR的整体过表达(++ OXTR)概括了PCOS的主要特征,包括高雄激素症、少排卵和多囊卵巢变化。卵巢病理表现为卵泡闭锁、囊变、出血和黄体形成不足。这些形态改变与严重的内分泌失调相吻合,表现为高催乳素血症、黄体生成素(LH)分泌抑制和黄体酮(P)缺乏,与++Oxtr雄性保持生育能力形成对比。在机制上,我们确定了oxtr -催乳素(PRL)-p-STAT3轴是PCOS发病的核心。与高泌乳素血症相对应的是,++Oxtr卵巢中核p-STAT3 (Tyr705)的持续激活——在妊娠期WT对照组中不存在——在影响排卵的同时上调卵泡生成基因(Lhcgr, Pgr, Leptin, Cyp17a1)。治疗干预用溴隐亭使催乳素和黄体酮水平正常化,部分恢复卵巢功能。值得注意的是,++Oxtr女性尽管保持苗条的表型,但仍出现以胰岛素抵抗和性腺肥胖为特征的代谢功能障碍。我们的研究结果表明,OXTR是高泌乳素血症PCOS发病机制的一个新的上游调节剂,提示溴隐亭可能对高泌乳素血症PCOS有治疗价值。这些见解为有针对性的多囊卵巢综合征干预开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


