2-Hydroxychalcone is a gonococcal-specific antimicrobial with activity against elongation factor Tu and butanediol dehydrogenase
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Aims
This study explored botanical extracts to guide discovery of alternative antimicrobials with activity against the multidrug-resistant bacterial pathogen Neisseria gonorrhoeae.
Materials and methods
In vitro antimicrobial activity was analyzed by agar dilution method and time-kill assays, while in vivo activity was evaluated in a mouse infection model. The antimicrobial target was identified by pull-down assays and validated by interaction studies with wild-type and targeted mutant proteins.
Key findings
The extract of Chinese Populus spp. propolis (CPP) displayed the most potent antigonococcal activity. Subsequently, galangin was identified as the most active compound in CPP. However, galangin showed no in vivo activity in the mouse infection model. Screening for active structural analogues of galangin resulted in the identification of 2-hydroxychalcone (2-HC), which showed a minimum inhibitory concentration (MIC) of 8–16 μM, and enhanced gonococcal clearance in the infection model. Pull-down experiments identified EF-Tu and butanediol dehydrogenase (Bdh) as putative 2-HC targets. Further binding analysis of 2-HC with recombinant purified proteins showed an equilibrium dissociation constant (KD) of 1.28 μM for EF-Tu and 5.97 μM for Bdh. Finally, 2-HC binding pockets on EF-Tu and Bdh were identified by molecular docking studies showing that 2-HC interacted with Glu260 of EF-Tu and Ser273 of Bdh, which was validated by mutagenesis studies. For Bdh, we furthermore demonstrated that 2-HC impacted its enzyme activity with and IC50 of 44–64 μM, resulting in perturbed NAD+/NADH ratios.
Significance
2-HC displays a bimodal antigonococcal mechanism, making it an interesting candidate for further development as antigonococcal therapy.
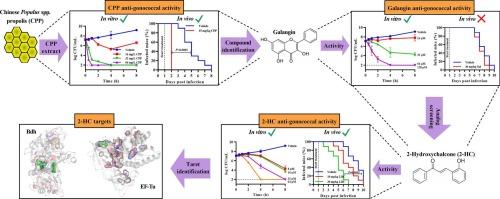
2-羟基查尔酮是一种淋球菌特异性抗菌药物,具有抗延伸因子Tu和丁二醇脱氢酶的活性。
目的:探索植物提取物,以指导发现具有抗多药耐药淋病奈瑟菌活性的替代抗菌剂。材料和方法:采用琼脂稀释法和时间杀伤法分析其体外抗菌活性,并采用小鼠感染模型评价其体内抗菌活性。通过拉下实验确定了抗菌靶点,并通过与野生型和靶向突变蛋白的相互作用研究进行了验证。主要发现:中国胡杨蜂胶提取物(CPP)的抗淋球菌活性最强。随后,高良姜素被鉴定为CPP中最具活性的化合物。然而,高良姜在小鼠感染模型中没有表现出体内活性。筛选高良姜活性结构类似物,鉴定出2-羟基查尔酮(2-HC),其最低抑制浓度(MIC)为8-16 μM,并增强了感染模型中淋球菌的清除率。下拉实验确定EF-Tu和丁二醇脱氢酶(Bdh)可能是2-HC的靶点。2-HC与重组纯化蛋白的进一步结合分析表明,EF-Tu的平衡解离常数(KD)为1.28 μM, Bdh的平衡解离常数为5.97 μM。最后,通过分子对接研究确定了EF-Tu和Bdh上的2-HC结合口袋,结果表明,2-HC与EF-Tu的Glu260和Bdh的Ser273相互作用,并通过诱变研究验证了这一点。对于Bdh,我们进一步证明了2-HC影响其酶活性,IC50为44-64 μM,导致NAD+/NADH比率受到干扰。意义:2-HC显示双峰抗淋球菌机制,使其成为进一步开发抗淋球菌治疗的有趣候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


