Linker Length and Composition within Disordered Binding Motifs Modulates the Avidity and Reversibility of a Multivalent Protein Interaction Switch
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
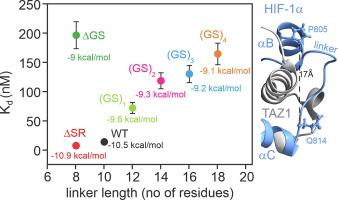
Intrinsically disordered proteins that mediate the cellular transcriptional response to hypoxia play important roles in regulating oxygen stress genes. The feedback inhibitor CITED2 operates a unidirectional switch that efficiently terminates the hypoxic response by displacing the C-terminal activation domain of the hypoxia-inducible factor HIF-1α from its complex with the TAZ1 domain of the transcriptional coactivators CBP and p300. Unidirectionality of the switch arises from subtle allosteric conformational changes in TAZ1 and from differences in the strength of thermodynamic coupling between the TAZ1-binding motifs in the multivalent HIF-1α and CITED2 activation domains. To investigate the role of binding cooperativity, we mutated a linker sequence in the HIF-1α activation domain to alter the thermodynamic coupling between its TAZ1-binding motifs. Linker mutations that enhance the affinity of HIF-1α for TAZ1 enable the HIF-1α activation domain to compete more effectively with bound CITED2. The wide range of mutants, which include insertion, deletion, substitution, and scrambling of residues in the linker, provide insights into the molecular basis for the exquisite tuning of the hypoxic switch. The TAZ1 binding affinity and consequent CITED2 competition enhancement depends both on the flexibility of the linker sequence (particularly the presence of glycine residues) and the unfavorable electrostatic interactions of a highly conserved arginine side chain in the center of the linker with an electropositive surface of TAZ1. The conservation of the linker length and sequence in all vertebrates suggests strong evolutionary pressure to tune HIF-1α binding affinity to be sub-optimal, to ensure unidirectionality of the hypoxic switch.
无序结合基序内的连接体长度和组成调节多价蛋白相互作用开关的快速性和可逆性。
内在无序蛋白介导细胞对缺氧的转录反应,在调节氧应激基因中发挥重要作用。反馈抑制剂CITED2操作一个单向开关,通过将缺氧诱导因子HIF-1α的c端激活域从其与转录共激活因子CBP和p300的TAZ1域的复合体中取代,有效地终止缺氧反应。这种开关的单向性源于TAZ1的微妙变构构象变化,以及多价HIF-1α和CITED2激活域中TAZ1结合基序之间热力学耦合强度的差异。为了研究结合协同性的作用,我们突变了HIF-1α激活域中的一个连接子序列,以改变其taz1结合基序之间的热力学耦合。连接体突变增强了HIF-1α对TAZ1的亲和力,使HIF-1α激活域能够更有效地与结合的CITED2竞争。广泛的突变,包括连接体中残基的插入、删除、替换和置乱,为精细调节缺氧开关的分子基础提供了见解。TAZ1的结合亲和力和随后的CITED2竞争增强取决于连接体序列的灵活性(特别是甘氨酸残基的存在)和连接体中心高度保守的精氨酸侧链与TAZ1的正电表面的不利静电相互作用。所有脊椎动物中连接子长度和序列的保守性表明,强大的进化压力将HIF-1α结合亲和力调整为次优,以确保缺氧开关的单向性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


