Cavidine alleviates paclitaxel-induced peripheral neuropathy by promoting mitochondrial autophagy through inhibiting PKM2-mediated histone lactylation
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Background
Chemotherapy-induced peripheral neuropathy (CIPN) is a debilitating adverse effect of chemotherapy, with limited therapeutic options due to unclear mechanisms. Cavidine (CAV), a natural alkaloid, has been shown to possess both anti-inflammatory and neuroprotective properties. However, further research is required to elucidate its role in CIPN and the underlying mechanisms by which it exerts its effects.
Purpose
To examine the therapeutic efficacy of CAV in relation to CIPN, with a view to elucidating its underlying mechanism, which is associated with mitophagy and PKM2-mediated histone lactylation.
Methods
The post-CAV treatment assessment included the evaluation of mechanical allodynia, thermal hyperalgesia, and footpad immunofluorescence. To identify the regulated pathways of CAV, RNA-seq and lactate metabolomics were performed. The evaluation of mitophagy was conducted through the utilization of immunofluorescence, along with transmission electron microscopy. In addition, the analysis of histone lactylation at H3K18la was undertaken. The use of molecular docking and biolayer interferometry assay confirmed the interactions between CAV-PKM2.
Results
CAV significantly alleviated both mechanical and thermal pain, as well as peripheral nerve injury, in mice suffering from CIPN. Mechanistically, CAV suppressed PKM2 activity, reducing lactate accumulation and histone H3K18 lactylation. This inhibition promoted mitochondrial autophagy, evidenced by decreased LC3B-II, upregulated PINK1/Parkin, and reduced p62, and promoted the integration of autophagosomes and lysosomes. Molecular docking and biolayer interferometry assay demonstrated high-affinity binding between CAV and the allosteric site of PKM2.
Conclusion
CAV alleviates CIPN by enhancing mitophagy via inhibition of PKM2-driven histone lactylation, thus providing a novel therapeutic strategy for CIPN.
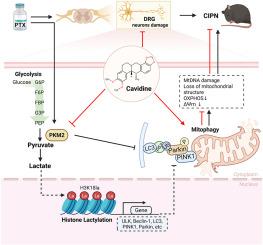
卡维定通过抑制pkm2介导的组蛋白乳酸化,促进线粒体自噬,减轻紫杉醇诱导的周围神经病变。
背景:化疗诱导的周围神经病变(CIPN)是化疗的一种使人衰弱的不良反应,由于机制不明确,治疗选择有限。Cavidine (CAV)是一种天然生物碱,具有抗炎和神经保护作用。然而,需要进一步的研究来阐明其在CIPN中的作用及其发挥作用的潜在机制。目的:研究CAV对CIPN的治疗效果,以阐明其与线粒体自噬和pkm2介导的组蛋白乳酸化有关的潜在机制。方法:cav治疗后的评估包括机械异常性痛、热痛觉过敏和足垫免疫荧光的评估。为了确定CAV的调控途径,我们进行了RNA-seq和乳酸代谢组学研究。利用免疫荧光法和透射电镜对线粒体自噬进行评价。此外,我们还对H3K18la的组蛋白乳酸化进行了分析。利用分子对接和生物层干涉法证实了CAV-PKM2之间的相互作用。结果:CAV可显著减轻CIPN小鼠的机械痛和热痛以及周围神经损伤。在机制上,CAV抑制PKM2活性,减少乳酸积累和组蛋白H3K18的乳酸化。这种抑制促进了线粒体自噬,表现为LC3B-II降低,PINK1/Parkin上调,p62降低,促进了自噬体和溶酶体的整合。分子对接和生物层干涉实验表明CAV与PKM2变构位点具有高亲和力结合。结论:CAV通过抑制pkm2驱动的组蛋白乳酸化来增强自噬,从而缓解CIPN,为CIPN的治疗提供了一种新的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


