Amorphous Titanosilicate Catalysts for Homolytic Oxidation of Cyclohexene under Mild Conditions
IF 1.1
4区 工程技术
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
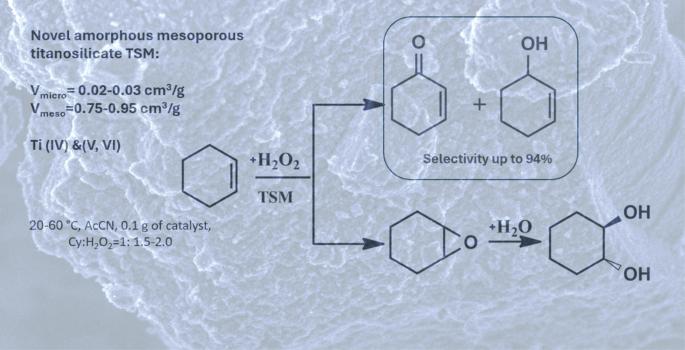
Catalytic properties that mesoporous amorphous titanosilicate catalysts differing in physicochemical characteristics exhibit in cyclohexene oxidation with hydrogen peroxide to a mixture consisting mainly of alcohol and ketone were studied. The cyclohexene conversion and selectivity of formation of oxidation products depend both on the physicochemical properties of the titanosilicates studied (nature of active sites, porosity, etc.) and on the reaction conditions. The total selectivity with respect to the enol + enone fraction of 94% at the maximal cyclohexene conversion of 55% was reached in the presence of TSMa titanosilicate (Si/Ti = 40).
无定形钛酸盐催化剂在温和条件下均溶氧化环己烯
研究了不同理化性质的介孔无定形钛酸盐催化剂在过氧化氢氧化环己烯生成醇酮混合物中的催化性能。环己烯的转化率和氧化产物形成的选择性取决于所研究的钛硅酸盐的物理化学性质(活性位点的性质、孔隙度等)和反应条件。TSMa钛酸盐(Si/Ti = 40)对烯醇+烯酮的总选择性为94%,最大环己烯转化率为55%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Petroleum Chemistry
工程技术-工程:化工
CiteScore
2.50
自引率
21.40%
发文量
102
审稿时长
6-12 weeks
期刊介绍:
Petroleum Chemistry (Neftekhimiya), founded in 1961, offers original papers on and reviews of theoretical and experimental studies concerned with current problems of petroleum chemistry and processing such as chemical composition of crude oils and natural gas liquids; petroleum refining (cracking, hydrocracking, and catalytic reforming); catalysts for petrochemical processes (hydrogenation, isomerization, oxidation, hydroformylation, etc.); activation and catalytic transformation of hydrocarbons and other components of petroleum, natural gas, and other complex organic mixtures; new petrochemicals including lubricants and additives; environmental problems; and information on scientific meetings relevant to these areas.
Petroleum Chemistry publishes articles on these topics from members of the scientific community of the former Soviet Union.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


