Dual-PROTACs based on natural product derivative potassium dehydrographolide succinate: design, synthesis, and antitumor activity of a novel EGFR degrader
Abstract
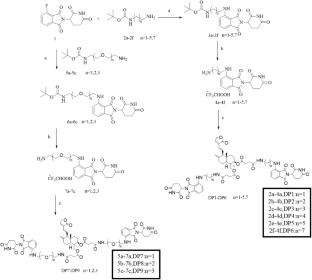
The epidermal growth factor receptor (EGFR) is overexpressed in various cancers and contributes to tumor progression and therapeutic resistance. Although EGFR-targeting small-molecule inhibitors are clinically available, their limited efficacy and acquired resistance pose major challenges. In this study, we designed and synthesized a novel class of dual proteolysis-targeting chimeras (PROTACs) incorporating the natural product derivative Potassium Dehydroandrographolide Succinate (PDS) as the protein of interest (POI) ligand. PDS was selected as the POI ligand due to its structural similarity to andrographolide, a natural compound known to inhibit EGFR signaling, suggesting that PDS may retain EGFR-binding potential despite lacking direct anti-tumor reports. Unlike conventional PROTACs, these molecules feature two CRBN E3 ligase ligands symmetrically attached via distinct linkers, thereby enhancing the likelihood of ternary complex formation and promoting more efficient EGFR degradation. Among the synthesized compounds, DP6 exhibited the most potent anti-proliferative activity in MCF-7 cells, with a 3.8-fold improvement over the parent PDS molecule. Western blotting confirmed that DP6 induced concentration-dependent EGFR degradation via the ubiquitin–proteasome system, suppressed downstream JAK2-STAT3 signaling, and promoted apoptosis. This study not only demonstrates the feasibility of utilizing structurally modified natural products as POI ligands, but also introduces a unique dual-ligand PROTAC architecture that may provide enhanced degradation potency for traditionally “undruggable” targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


