Analytical Method Development Strategy for Controlling Two New Nitrosamine Drug Substance Related Impurities (NDSRIs) in a Pharmaceutical Drug Product for Treatment of a Rare Disease
Abstract
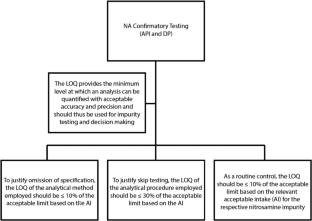
N-Nitrosamines are raised as having health consequences by the European Medical Agencies ICH M7(R2) guidance on Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. It has been suggested that nitrosamines have carcinogenic potential, and efforts should be made to control them at acceptable and safe levels in drug substances (DS). The detection of nitrosamine drug substance related impurities (NDSRIs) historically has encountered considerable challenges in establishing compound specific acceptable intake (AI) limits. Often, a default low AI limit of 18 ng/day was assigned to the NDSRIs due to the lack of their toxicology data, prior to the introduction of the current Carcinogenic Potency Categorization Approach (CPCA). Developing analytical methods to quantify NDSRIs posed a challenge due to an extremely low Quantitation Limit (QL), not exceeding 10% of the AI limit based on a conservative Threshold of Toxicological Concern (TTC) approach. This work presents the method development strategy for two complex diastereomeric NDSRIs in miglustat, a recently commercialized rare disease drug. A predictive chemistry for NDSRI formation and organic synthesis earlier was followed by in silico assessments of potential mutagenicity and carcinogenicity. This paper describes the development of a highly sensitive analytical method with a QL of 6.9 ppb for the combined NDSRIs, in alignment with the regulatory recommendation at the time of this work. The validated method was employed for confirmatory testing of miglustat batches for the combined NDSRIs. The results were below QL, and no routine NDSRI testing was required for future drug product (DP) batch release. This novel approach may offer valuable insights into the numerous molecules in development that will now require rigorous nitrosamine assessment.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


