Synthesis of Conjugates of N-(Purin-6-yl)-6-aminohexanoic Acid with 4-Amino-1-aryl-5-oxoprolines
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
(2S,4S)-4-Amino-1-aryl-5-oxoprolines were synthesized by nucleophilic substitution of the bromine atom in dimethyl (2S,4RS)-4-bromo-N-phthaloylglutamate under the action of difluorosubstituted anilines followed by isolation of (2S,4S)-diastereomers and removal of protecting groups; subsequent coupling of their methyl esters with N-(purin-6-yl)-6-aminohexanoic acid and saponification of ester groups led to the formation of target conjugates. The saponification of ester groups was accompanied by minor epimerization.
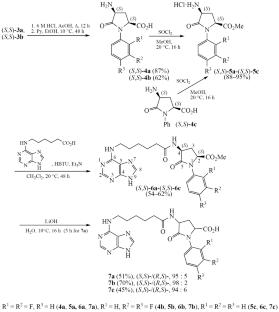
N-(嘌呤-6-基)-6-氨基己酸与4-氨基-1-芳基-5-氧脯氨酸缀合物的合成
在二氟取代苯胺的作用下,将(2S,4RS)-4-溴- n -邻苯酞谷氨酸二甲基中的溴原子亲核取代,分离(2S,4S)-非对映体并去除保护基团,合成了(2S,4S)-4-氨基-1-芳基-5-氧脯氨酸;随后,它们的甲酯与N-(嘌呤-6-酰基)-6-氨基己酸偶联,酯基皂化形成目标偶联物。酯基的皂化反应伴随着少量的外聚反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


