Chemodivergent Pd-Catalyzed Cyanation of 5-Iodo-1,2,3-triazoles
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A divergent approach to 5-cyanotriazoles and triazolo[1,5-a]quinolines based on Pd-catalyzed cyanation of 2-(5-iodotriazolyl)phenylacetic acid derivatives has been developed. Chemoselectivity is controlled by the choice of cyanide source. Thus, CuCN leads only to the replacement of iodine by a nitrile moiety, while KCN induces further cyclization of the cyanation product via intramolecular condensation. The method is applicable to the preparation of both types of heterocyclic compounds in good yields (63–88%).
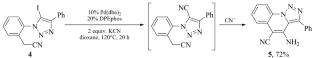
化学发散pd催化5-碘-1,2,3-三唑的氰化反应
基于pd催化的2-(5-碘三唑)苯基乙酸衍生物的氰化反应,提出了一种不同的方法来制备5-氰三唑和三唑[1,5- A]喹啉。化学选择性是由氰化物来源的选择控制的。因此,CuCN只导致碘被腈部分取代,而KCN通过分子内缩合诱导氰化产物进一步环化。该方法适用于两类杂环化合物的制备,收率均为63 ~ 88%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


