Synthesis of New 1,3a,6,6a-Tetraazapentalene Derivatives under Cadogan Reaction Conditions
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
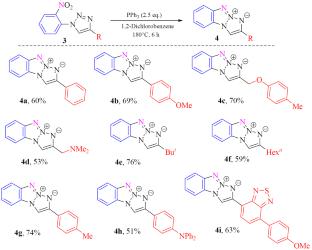
A convenient synthetic approach has been developed for novel monobenzotriazoles incorporating a mesoionic 1,3a,6,6a-tetraazapentalene core. The method involves a Cu(I)-catalyzed (3+2) cycloaddition of ortho-nitrophenyl azide to terminal aryl(alkyl)acetylenes, followed by an intramolecular cyclization under Cadogan-type deoxygenation conditions. The process can be successfully performed in a one-pot fashion, affording the target benzo[d][1,2,3]triazolo[1,2-a]triazole derivatives in good yields.
Cadogan反应条件下新1,3a,6,6a-四氮杂二烯衍生物的合成
提出了一种以1,3a,6,6 A -四氮杂二烯为介离子核的新型单苯并三唑的简便合成方法。该方法包括Cu(I)催化邻硝基苯基叠氮化物(3+2)环加成到末端芳基(烷基)乙炔上,然后在cadogan型脱氧条件下进行分子内环化。该工艺可以在一锅方式下成功地进行,为目标苯并[d][1,2,3]三唑[1,2-a]三唑衍生物提供了良好的收率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


