TCEB2 promotes M2 polarization of macrophages in triple negative breast cancer by mediating ubiquitination degradation of Slit2 through recruiting NEDD4
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Background
M2-polarized tumor-associated macrophage infiltration is a key risk factor for poor prognosis in triple-negative breast cancer (TNBC). This study investigated the role of transcription elongation factor B polypeptide 2 (TCEB2) in regulating M2 macrophage polarization during TNBC development.
Methods
The expression of gene or protein was tested by qRT-PCR, western blot, and IHC. CCK8, colony formation, wound healing, and Transwell assays were used to evaluate TNBC cell malignant behaviors, including cell viability, proliferation, migration, and invasion. The secretion levels of cytokines were detected by ELISA. Ubiquitin-based IP assays were used to detect Slit2 ubiquitination. The combined relation between TCEB2, Slit2, and NEDD4 was investigated using Co-IP assay.
Results
Our results demonstrated that M2 macrophage polarization was activated in TNBC, which might be related to TCEB2 upregulation. TCEB2 knockdown reduced TNBC cell growth, migration, invasion, and their ability to induce M2 macrophage polarization. Mechanistically, TCEB2 mediated Slit2 K63 ubiquitination degradation in TNBC by interacting with NEDD4. As expected, the inhibitory effect of TCEB2 silencing on the ability of TNBC cells to induce M2 macrophage polarization was reversed by Slit2 knockdown. Finally, TCEB2 knockdown inhibited TNBC tumor growth and TNBC-induced M2 macrophage polarization in vivo.
Conclusion
TCEB2 upregulation promoted TNBC-induced M2 macrophage polarization to accelerate TNBC development by mediating Slit2 K63-ubiquitination degradation through interacting with NEDD4.
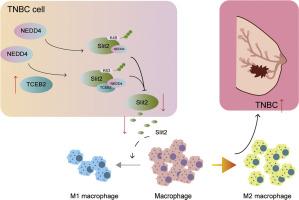
TCEB2通过募集NEDD4介导Slit2泛素化降解,促进三阴性乳腺癌巨噬细胞M2极化
m2极化肿瘤相关巨噬细胞浸润是三阴性乳腺癌(TNBC)预后不良的关键危险因素。本研究探讨了转录延伸因子B多肽2 (TCEB2)在TNBC发生过程中调控M2巨噬细胞极化的作用。方法采用qRT-PCR、western blot和免疫组化检测基因或蛋白的表达。CCK8、菌落形成、伤口愈合和Transwell检测用于评估TNBC细胞的恶性行为,包括细胞活力、增殖、迁移和侵袭。ELISA法检测细胞因子分泌水平。基于泛素的IP法检测Slit2泛素化。采用Co-IP法研究TCEB2、Slit2和NEDD4的联合关系。结果TNBC中M2巨噬细胞极化被激活,这可能与TCEB2上调有关。TCEB2敲低可降低TNBC细胞的生长、迁移、侵袭,并降低其诱导M2巨噬细胞极化的能力。机制上,TCEB2通过与NEDD4相互作用介导TNBC中Slit2 K63泛素化降解。正如预期的那样,TCEB2沉默对TNBC细胞诱导M2巨噬细胞极化能力的抑制作用被Slit2敲除逆转。最后,TCEB2敲除抑制TNBC肿瘤生长和TNBC诱导的M2巨噬细胞极化。结论tceb2上调通过与NEDD4相互作用介导Slit2 k63泛素化降解,促进TNBC诱导的M2巨噬细胞极化,加速TNBC的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


