Assessing the mechanism of osteosarcoma induced by long-term PET exposure: prediction from combined network toxicology, machine learning and molecular docking
IF 3.5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Objective
Polyethylene terephthalate (PET) has emerged as a focal point in addressing global pollution and a critical environmental issue due to its potential health hazards. However, its role in the pathogenesis of osteosarcoma (OS) and the underlying molecular mechanisms remain largely unexplored, further highlighting the necessity of assessing its molecular toxicity.
Methods
This study integrated network toxicology, machine learning, molecular docking, and CIBERSORT-based immune infiltration analysis to systematically investigate the potential impact of PET exposure on contracting OS, elucidating its biological functions, signaling mechanisms, and immune microenvironment. Molecular docking was further applied to characterize the binding properties of PET with hub proteins, and potential therapeutic agents for OS were predicted.
Results
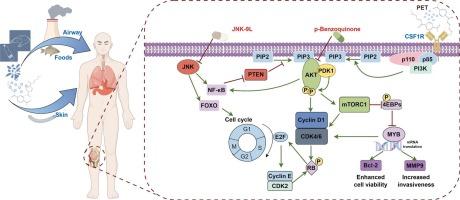
We identified 12 potential key targets of OS associated with PET exposure and, through machine learning models, selected six hub genes (i.e., BCAT1, CDK4, CSF1R, CXCR4, MYB, and PRTN3). Gene Ontology (GO) and kyoto encyclopedia of genes and genomes (KEGG) analyses were conducted to elucidate the roles of these genes in biological processes, cellular components, molecular functions, and signaling pathways. Molecular docking results revealed that PET exhibits high specificity in binding to these hub genes, particularly by interacting with CSF1R (−8.312 kcal/mol), potentially activating the PI3K-Akt signaling pathway and modulating the OS immune microenvironment to promote tumor progression through multiple mechanisms. Furthermore, drug prediction analysis identified p-Benzoquinone and JNK-9L as potential therapeutic candidates for OS.
Conclusion
This study reveals that PET may play a critical role in OS development by regulating hub genes and signaling pathways. Molecular docking analysis demonstrates that PET can tightly bind to specific target proteins, suggesting a potential molecular mechanism underlying OS progression. These findings provide a scientific basis for further evaluating PET-related health risks and offer theoretical support for the development of future prevention and treatment strategies.
评估PET长期暴露诱发骨肉瘤的机制:基于网络毒理学、机器学习和分子对接的联合预测
目的聚对苯二甲酸乙二醇酯(PET)因其潜在的健康危害已成为解决全球污染和关键环境问题的焦点。然而,其在骨肉瘤(OS)发病机制中的作用及其潜在的分子机制在很大程度上仍未被探索,进一步强调了评估其分子毒性的必要性。方法采用网络毒理学、机器学习、分子对接、基于cibersort的免疫浸润分析等方法,系统研究PET暴露对感染OS的潜在影响,阐明其生物学功能、信号机制和免疫微环境。分子对接进一步表征PET与枢纽蛋白的结合特性,并预测潜在的OS治疗剂。我们确定了与PET暴露相关的12个潜在的OS关键靶点,并通过机器学习模型选择了6个中心基因(即BCAT1、CDK4、CSF1R、CXCR4、MYB和PRTN3)。通过基因本体(GO)和京都基因与基因组百科全书(KEGG)分析,阐明了这些基因在生物过程、细胞成分、分子功能和信号通路中的作用。分子对接结果显示,PET与这些枢纽基因的结合具有很高的特异性,特别是通过与CSF1R的相互作用(−8.312 kcal/mol),可能激活PI3K-Akt信号通路,调节OS免疫微环境,通过多种机制促进肿瘤进展。此外,药物预测分析确定对苯醌和JNK-9L是OS的潜在治疗候选者。结论PET可能通过调控中枢基因和信号通路在OS发育中发挥重要作用。分子对接分析表明,PET可以与特异性靶蛋白紧密结合,提示OS进展的潜在分子机制。这些发现为进一步评价pet相关健康风险提供了科学依据,并为今后制定防治策略提供了理论支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Bone Oncology
ONCOLOGY-
CiteScore
7.20
自引率
2.90%
发文量
50
审稿时长
34 days
期刊介绍:
The Journal of Bone Oncology is a peer-reviewed international journal aimed at presenting basic, translational and clinical high-quality research related to bone and cancer.
As the first journal dedicated to cancer induced bone diseases, JBO welcomes original research articles, review articles, editorials and opinion pieces. Case reports will only be considered in exceptional circumstances and only when accompanied by a comprehensive review of the subject.
The areas covered by the journal include:
Bone metastases (pathophysiology, epidemiology, diagnostics, clinical features, prevention, treatment)
Preclinical models of metastasis
Bone microenvironment in cancer (stem cell, bone cell and cancer interactions)
Bone targeted therapy (pharmacology, therapeutic targets, drug development, clinical trials, side-effects, outcome research, health economics)
Cancer treatment induced bone loss (epidemiology, pathophysiology, prevention and management)
Bone imaging (clinical and animal, skeletal interventional radiology)
Bone biomarkers (clinical and translational applications)
Radiotherapy and radio-isotopes
Skeletal complications
Bone pain (mechanisms and management)
Orthopaedic cancer surgery
Primary bone tumours
Clinical guidelines
Multidisciplinary care
Keywords: bisphosphonate, bone, breast cancer, cancer, CTIBL, denosumab, metastasis, myeloma, osteoblast, osteoclast, osteooncology, osteo-oncology, prostate cancer, skeleton, tumour.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


