Antimicrobial peptide mechanism of action on S. aureus membranes determined by in vivo solid-state NMR
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Staphylococcus aureus (S. aureus) is a Gram-positive pathogenic bacterium and a major cause of nosocomial infections. Between 20 % and 50 % of S. aureus strains are resistant to a wide range of antibiotics. DMS-DA6-NH2 (DA6) is a novel antimicrobial peptide (AMP) that exhibits high efficacy against various bacterial strains, particularly S. aureus, by disrupting its membrane through an as-yet-unknown mechanism. We employed in vivo 2H solid state Nuclear Magnetic Resonance (NMR) to investigate the mode of action of AMPs on deuterated bacteria. This technique provides insights into membrane order and its changes with increasing AMP concentration. Our results enabled us to compare the mechanism of DA6 with those of AMPs with established modes of action. We found that DA6 induces pore formation in the membrane of S. aureus. This protocol serves as a template for determining the mechanisms of action of other peptides, an essential step for developing and patenting such drugs for the treatment of human diseases.
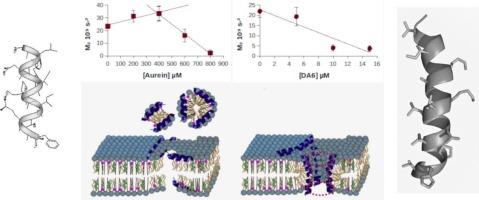
体内固体核磁共振测定抗菌肽对金黄色葡萄球菌膜的作用机制
金黄色葡萄球菌(金黄色葡萄球菌)是一种革兰氏阳性致病菌,也是医院感染的主要原因。20%至50%的金黄色葡萄球菌菌株对多种抗生素具有耐药性。DMS-DA6-NH2 (DA6)是一种新型抗菌肽(AMP),通过一种未知的机制破坏其膜,对各种细菌菌株,特别是金黄色葡萄球菌表现出高效率。我们利用2H固态核磁共振(NMR)研究了AMPs对氘化细菌的作用模式。这项技术提供了对膜序及其随AMP浓度增加而变化的见解。我们的结果使我们能够将DA6的机制与具有既定作用模式的amp的机制进行比较。我们发现DA6可以诱导金黄色葡萄球菌的膜形成孔。该协议可作为确定其他多肽作用机制的模板,这是开发用于治疗人类疾病的此类药物并为其申请专利的重要步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


