Molecular insights into the transport and toxicity of 6-PPD: Interactions with human serum albumin and alpha-glucosidase
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The rubber antioxidant, N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6-PPD), as an emerging pollutant, is receiving more and more attention recently. This study investigated the intermolecular interactions of 6-PPD with two key biological macromolecules, human serum albumin (HSA) and alpha-glucosidase (AG), to understand the transport and toxic effects of 6-PPD. Using multiple spectroscopic methods and molecular docking technology, the results demonstrated that 6-PPD could bind to both HSA and AG, thereby inducing fluorescence quenching and conformational changes in both proteins. The binding constants were determined to be (5.93 ± 0.20) × 105 and (3.17 ± 0.15) × 104 L mol−1 respectively for HSA-6-PPD and AG-6-PPD systems at 298 K, revealing strong binding affinities. Molecular docking identified specific binding sites and non-covalent interactions of the two systems. MD and Energy decomposition analysis revealed the dynamics conformational changes of the complexes and identified van der Waals and electrostatic interactions as primary binding drivers for both systems, while polar solvation energy impeded complex formation. TYR161, ILE142, and TYR138 dominated HSA-6-PPD stabilization, whereas AG-6-PPD was driven by hydrophobic interactions with TRP1369 and VAL1373, with ARG1377 incurring substantial desolvation penalties. Synchronous fluorescence and circular dichroism spectroscopy indicated that 6-PPD binding did not disrupt the microenvironment of Tyr and Trp residues in HSA and AG, while induced structural alterations in HSA and AG that could affect their physiological function. In-vitro tests showed that 6-PPD inhibited AG activity in a dose-dependent manner, with an IC50 of 8.22 ± 0.44 μmol L−1. ADMET and PASS online tools was used to predict physicochemical properties and multiorgan toxicity. This work provided insights into the transport and molecular toxicity of 6-PPD, highlighting the adverse biological effects associated with this common rubber additive.
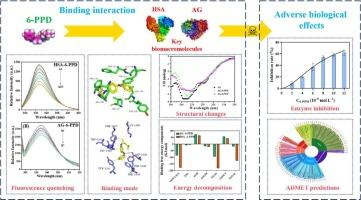
6-PPD转运和毒性的分子洞察:与人血清白蛋白和α -葡萄糖苷酶的相互作用
橡胶抗氧化剂N-(1,3-二甲基丁基)-N′-苯基-对苯二胺(6-PPD)作为一种新兴的污染物,近年来受到越来越多的关注。本研究探讨了6-PPD与人血清白蛋白(HSA)和α -葡萄糖苷酶(AG)这两个关键生物大分子的分子间相互作用,以了解6-PPD的转运和毒性作用。利用多种光谱方法和分子对接技术,结果表明6-PPD可以同时与HSA和AG结合,从而引起两种蛋白的荧光猝灭和构象变化。在298 K下,HSA-6-PPD和AG-6-PPD的结合常数分别为(5.93±0.20)× 105和(3.17±0.15)× 104 L mol - 1,具有较强的结合亲和力。分子对接确定了两个体系的特异性结合位点和非共价相互作用。MD和能量分解分析揭示了配合物的动态构象变化,并确定范德华和静电相互作用是两种体系的主要结合驱动因素,而极性溶剂化能阻碍配合物的形成。TYR161、ILE142和TYR138主导了HSA-6-PPD的稳定,而AG-6-PPD是由与TRP1369和VAL1373的疏水相互作用驱动的,而ARG1377会产生大量的溶解效应。同步荧光和圆二色光谱显示,6-PPD结合不会破坏HSA和AG中Tyr和Trp残基的微环境,但会引起HSA和AG的结构改变,从而影响其生理功能。体外实验表明,6-PPD对AG活性的抑制呈剂量依赖性,IC50为8.22±0.44 μmol L−1。ADMET和PASS在线工具用于预测理化性质和多器官毒性。这项工作提供了对6-PPD的转运和分子毒性的见解,突出了与这种常见橡胶添加剂相关的不利生物效应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


