Kinetics and mechanism of oxidation of L-cysteine, DL-homocysteine and glutathione by trans-(diaqua)(salen)manganese(III) complex in aqueous medium: Structure optimization at the DFT level: Influence of externally added copper(II)
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The kinetics of oxidation of L-cysteine (cys) by trans-MnIII(salen)(OH2)2+ (H2salen = N,N′-bis(salicylidene)ethane-1,2-diamine) is studied at 30.0–45.0 °C, 1.90 ≤ pH ≤ 7.46, I = 0.3 mol dm−3, and the same is investigated for DL-homocysteine (Hcys) at 35 °C for comparison. Similarly, kinetic studies are performed for glutathione (GSH) at 30.0–45. 0 °C, 4.35 ≤ pH ≤ 7.46. The product analysis indicated the formation of corresponding disulphides, and MnIII is reduced to MnII. The same products are also formed when the oxidation is carried out in the presence of externally added Cu2+ ions. Although the oxidation is moderately catalyzed in the presence of Cu2+ ions but it is retarded by the chelating ligand EDTA. The stoichiometric ratio 1:1 (X = cys, Hcys, and GSH). The reaction proceeds via fast equilibrium pre-association inner-sphere complexes between trans-MnIII(salen)(OH2)2+ and X, followed by very slow intramolecular electron transfer steps. The kinetic parameters for various steps for cys, Hcys, and GSH are presented. The results indicate an outer-sphere electron transfer mechanism for the reduction of MnIII to MnII. The DFT-optimized structures supported the carboxylate mode of binding and the ground state structural trans effect for all the species. In addition, it supports a proton-controlled electron transfer process (PCET).
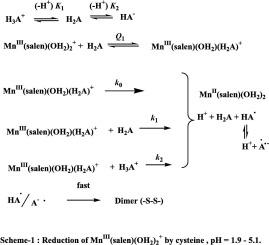
水介质中反式(双水)(salen)锰(III)配合物氧化l -半胱氨酸、dl -同型半胱氨酸和谷胱甘肽的动力学和机理:DFT水平上的结构优化:外加铜(II)的影响
研究了反式mniii (salen)(OH2)2+ (H2salen = N,N′-双(水杨基)乙烷-1,2-二胺)在30.0 ~ 45.0℃,1.90≤pH≤7.46,I = 0.3 mol dm−3条件下l -同型半胱氨酸(Hcys)在35℃条件下的氧化动力学。同样,在30.0-45℃对谷胱甘肽(GSH)进行动力学研究。0℃,4.35≤pH≤7.46。产物分析表明生成了相应的二硫化物,MnIII被还原为MnII。当在外部添加Cu2+离子的情况下进行氧化也会形成相同的产物。虽然在Cu2+离子存在下,氧化反应有一定的催化作用,但螯合配体EDTA对氧化反应有一定的阻滞作用。化学计量比∆MnIIIsalenH2O2+:∆X= 1:1 (X = cys, Hcys, GSH)。反应通过trans-MnIII(salen)(OH2)2+和X之间的快速平衡预结合球内配合物进行,然后是非常缓慢的分子内电子转移步骤。介绍了cys、hys和GSH在不同步骤中的动力学参数。结果表明,MnIII还原为MnII存在一个外球电子转移机制。dft优化的结构支持羧酸结合模式和基态结构反效应。此外,它支持质子控制的电子转移过程(PCET)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


