Synthesis of Cu/SiO2 catalysts via ionic exchange: Influence of copper loading on chemical structure and alcohol dehydrogenation
IF 4.7
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The study analyzes the effect of copper content in Cu/SiO2 catalysts (0.3–3.9 % w/w) obtained by the ion exchange method, comparing them with a catalyst prepared by wet impregnation (3.8 % w/w). Several characterization techniques, including XRD, DRUV-Vis, FTIR, and XPS, as well as a study of pyridine adsorption by FTIR, were employed to elucidate the structural and catalytic properties of the catalyst series. Results show that the copper-support interaction varies with concentration: at low concentrations, copper ions are effectively grafted onto the support, whereas at higher concentrations, a partial deposition of hydroxo-ammoniacal complexes begins as ion exchange reaches its limit. Textural properties of the support remain essentially unchanged during synthesis. DRUV-Vis and XPS spectra indicate the presence of dispersed Cu2+ species on the support surface, with the Cu/Si intensity ratio growing with copper loading. Catalytic activity increases with copper content up to a maximum, attributed to the formation of SiO–Cu–O–Cu–OSi and small Cu aggregates active sites. The materials exhibit strong selectivity to dehydrogenation reactions, with higher activity towards 2-propanol than ethanol. The paper explains the relationship between copper content, catalyst structure, and catalytic performance in Cu/SiO2 catalysts.
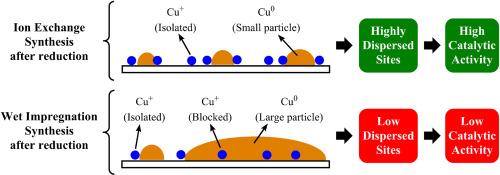
离子交换法合成Cu/SiO2催化剂:铜负载对化学结构和醇脱氢的影响
研究分析了铜含量对离子交换法制备的Cu/SiO2催化剂(0.3 ~ 3.9% w/w)的影响,并与湿浸渍法制备的催化剂(3.8% w/w)进行了比较。利用XRD、DRUV-Vis、FTIR、XPS等表征技术,以及FTIR对吡啶的吸附研究,对该系列催化剂的结构和催化性能进行了研究。结果表明,铜-载体的相互作用随浓度的变化而变化:在低浓度下,铜离子有效地接枝到载体上,而在高浓度下,随着离子交换达到极限,羟基-氨配合物开始部分沉积。在合成过程中,载体的纹理性质基本保持不变。DRUV-Vis和XPS光谱表明,随着铜的加载,载体表面存在分散的Cu2+物质,Cu/Si强度比增大。催化活性随着铜含量的增加而增加,达到最大值,这是由于SiO-Cu-O-Cu-OSi和小的Cu聚集活性位点的形成。该材料对2-丙醇脱氢反应具有较强的选择性,对2-丙醇的脱氢活性高于乙醇。本文阐述了Cu/SiO2催化剂中铜含量、催化剂结构和催化性能之间的关系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


