Mechanism-guided discovery of chromene-chalcone hybrids targeting PKM2 and microtubules for breast cancer therapy
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
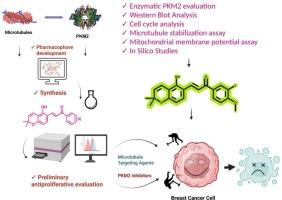
This study presents the rational pharmacophore design, synthesis, and biological evaluation of new chromene-chalcone hybrids (CCHs) with dual mechanisms involving pyruvate kinase M2 (PKM2) inhibition and microtubule stabilization for their potential as anticancer agents. The synthetic route involved the formation of a chromene aldehyde 29 via an oxa-Michael addition followed by intramolecular cyclization, which subsequently underwent Claisen Schmidt condensation to afford the final chalcone derivatives. Among the synthesized compounds (30a-30o), 30o emerged as the most promising candidate, exhibiting potent anticancer activity through dual targeting of PKM2 and the microtubule network. Compound 30o demonstrated a significant antiproliferative effect against MCF-7 breast cancer cells, with an IC₅₀ value of 10.2 ± 0.07 μM, and showed PKM2 enzymatic inhibition with an IC₅₀ of 0.363 ± 0.12 μM, as confirmed through enzymatic assays and protein expression. Cell cycle analysis revealed 30o induced G2/M phase arrest and microtubule-stabilizing activity. Furthermore, molecular modeling studies revealed its binding mode and strong interactions within the PKM2 active site and taxol binding site of tubulin, supporting the experimental findings. ADMET profiling predicted favorable pharmacokinetic and drug-likeness properties, highlighting its potential as a lead compound. Together, these findings underscored compound 30o as a dual-acting anticancer agent, simultaneously targeting cancer metabolism and cytoskeletal integrity, and offered a promising scaffold for further development in breast cancer therapeutics.
靶向PKM2和微管的铬-查尔酮杂合体用于乳腺癌治疗的机制引导发现
本研究介绍了一种新的具有抑制丙酮酸激酶M2 (PKM2)和微管稳定双重机制的铬-查尔酮杂合体(CCHs)的合理药效团设计、合成和生物学评价。该合成路线包括通过oxa-Michael加成形成铬醛29,然后进行分子内环化,随后进行Claisen Schmidt缩合以获得最终的查尔酮衍生物。在合成的化合物(30a-30o)中,30o是最有希望的候选化合物,通过双重靶向PKM2和微管网络表现出强大的抗癌活性。化合物300对MCF-7乳腺癌细胞具有显着的抗增殖作用,IC₅₀值为10.2±0.07 μM,并且通过酶测定和蛋白质表达证实,化合物300显示出PKM2的酶抑制作用,IC₅₀值为0.363±0.12 μM。细胞周期分析显示30o诱导G2/M相阻滞和微管稳定活性。此外,分子模型研究揭示了其结合模式以及在微管蛋白PKM2活性位点和紫杉醇结合位点之间的强相互作用,支持了实验结果。ADMET分析预测了良好的药代动力学和药物相似特性,突出了其作为先导化合物的潜力。总之,这些发现强调了化合物300作为一种双重作用的抗癌药物,同时针对癌症代谢和细胞骨架完整性,并为乳腺癌治疗的进一步发展提供了一个有希望的支架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


