Structure-guided design of a potent focal adhesion kinase (FAK) degrader via ternary complex modeling and molecular dynamics simulation
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Focal adhesion kinase (FAK) functions as a critical regulator of integrin-mediated signaling, orchestrating tumor progression and metastasis via both kinase-dependent enzymatic activity and kinase-independent scaffolding functions. Herein, we report the identification of a potent FAK-targeting PROTAC 9c via structure-guided ternary complex modeling to optimize linker geometry. In MDA-MB-231 cells, 9c demonstrated subnanomolar FAK degradation potency (DC50 = 3.6 nM), outperforming its parental inhibitor in suppressing cell proliferation, colony formation, migration and invasion. Moreover, 9c synergized with cisplatin to enhance chemosensitivity. Mechanistic studies revealed that 9c induces FAK degradation via a VHL-dependent ubiquitin-proteasome pathway. Notably, molecular dynamics simulations confirmed the formation of a stable FAK-9c-VHL ternary complex, rationalizing the linker design strategy. Together, this study establishes a structure-guided PROTAC design paradigm for efficient linker optimization, with 9c serving as a promising lead compound for targeting FAK-driven malignancies.
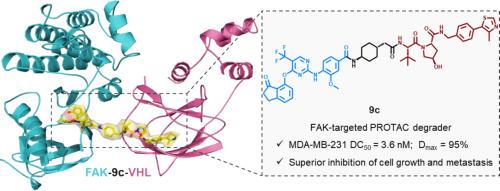
基于三元配合物模型和分子动力学模拟的高效黏附激酶(FAK)降解物结构导向设计
局灶黏附激酶(FAK)作为整合素介导的信号传导的关键调节因子,通过激酶依赖的酶活性和激酶独立的支架功能协调肿瘤的进展和转移。在此,我们报告了一个有效的fak靶向PROTAC 9c通过结构导向三元复杂建模来优化连接器的几何形状。在MDA-MB-231细胞中,9c表现出亚纳摩尔的FAK降解能力(DC50 = 3.6 nM),在抑制细胞增殖、集落形成、迁移和侵袭方面优于亲本抑制剂。此外,9c与顺铂协同作用可提高化疗敏感性。机制研究表明,9c通过vhl依赖的泛素-蛋白酶体途径诱导FAK降解。值得注意的是,分子动力学模拟证实了稳定的FAK-9c-VHL三元配合物的形成,使连接剂设计策略合理化。总之,本研究建立了一种结构导向的PROTAC设计范式,用于有效的连接子优化,其中9c作为靶向fak驱动的恶性肿瘤的有希望的先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


