Contribution of copper(II) and zinc(II) to the electrochemical and biological properties of 5-nitroimidazole coordination compounds. An experimental and theoretical approach
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
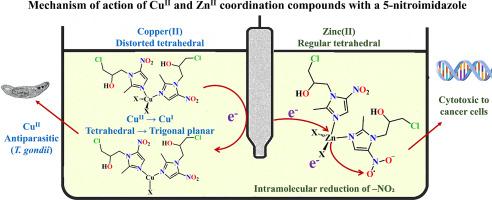
Coordination compounds of copper(II) and zinc(II) with the 5-nitroimidazole derivatives 1-(2-chloroethyl)-2-methyl-5-nitroimidazole (cenz) and 1-(3-chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole (onz) have shown antiparasitic activity against Toxoplasma gondii. This study combines electrochemical and theoretical approaches to evaluate the role of the metal ion in the formation and stability of redox species potentially involved in their mechanism of action. The free ligands exhibited oxidation and reduction peaks corresponding to the formation of nitro radical anion and hydroxylamine species, associated with their biological activity. In the coordination compounds, the metal ion influenced these processes by different mechanisms. Theoretical analysis revealed that the nitro radical anion can be stabilized by intramolecular hydrogen bonding or by lone pair···π hole interactions. In the copper(II) complexes, these interactions were further enhanced by a redox-driven structural rearrangement in which the coordination geometry shifts from distorted tetrahedral to trigonal planar upon CuII/CuI reduction. This geometrical change promotes stabilization of the nitro radical and facilitates redox cycling, which may contribute to antiparasitic activity. In contrast, the zinc(II) complexes maintain a regular tetrahedral geometry and present an intramolecular redox mechanism, where the nitro group is reduced via a transient state. To explore their potential anticancer activity, in vitro assays were conducted against HeLa, HCT-15 and A549 human cancer cell lines and L929 healthy fibroblast. Additionally acute toxicity studies were performed. The results support the potential of these coordination compounds as antiparasitic and anticancer agents, highlighting the key role of metal-induced structural and electronic effects into modulating biological activity.
铜(II)和锌(II)对5-硝基咪唑配位化合物电化学和生物学性质的贡献。一个实验和理论的方法
铜(II)和锌(II)与5-硝基咪唑衍生物1-(2-氯乙基)-2-甲基-5-硝基咪唑(cenz)和1-(3-氯-2-羟丙基)-2-甲基-5-硝基咪唑(onz)的配位化合物对刚地弓形虫具有抗寄生活性。本研究结合电化学和理论方法来评估金属离子在氧化还原物质的形成和稳定性中的作用,这些氧化还原物质可能参与了它们的作用机制。游离配体表现出氧化还原峰,与硝基阴离子和羟胺的形成相对应,与它们的生物活性有关。在配位化合物中,金属离子通过不同的机制影响这些过程。理论分析表明,硝基阴离子可以通过分子内氢键或孤对···π空穴相互作用来稳定。在铜(II)配合物中,氧化还原驱动的结构重排进一步增强了这些相互作用,在CuII/CuI还原时,配位几何从扭曲的四面体转变为三角形平面。这种几何变化促进了硝基的稳定,促进了氧化还原循环,这可能有助于抗寄生虫活性。相比之下,锌(II)配合物保持规则的四面体几何形状,并呈现分子内氧化还原机制,其中硝基通过瞬态还原。为了探索其潜在的抗癌活性,我们对HeLa、HCT-15和A549人癌细胞系和L929健康成纤维细胞进行了体外实验。此外,还进行了急性毒性研究。这些结果支持了这些配合物作为抗寄生虫和抗癌剂的潜力,突出了金属诱导的结构和电子效应在调节生物活性方面的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


