Inhibition of human and rat placental 3β-hydroxysteroid dehydrogenases by gallates: Combined experimental, 3D-QSAR, and in silico analysis
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Gallates are extensively utilized as additives in food, pharmaceuticals, and cosmetics. Despite their widespread use, their impact on the endocrine system remains poorly understood. This study investigates the potential interaction of gallates with 3β-hydroxysteroid dehydrogenase (3β-HSD), a critical enzyme in placental progesterone synthesis. The inhibitory effects and structure-activity relationship (SAR) and 3D-QSAR of nine gallates on human (h3β-HSD1) and rat (r3β-HSD4) placental enzymes were evaluated. Results revealed that the inhibitory potency of gallates on h3β-HSD1 was dependent on the carbon-chain length in the alcohol moiety. Propyl, butyl, hexyl, octyl, and dodecyl gallates exhibited mixed inhibition with IC50 values of 75.16, 64.59, 25.44, 19.56, and 5.99 μM, respectively, while gallic acid, methyl, ethyl, and cetyl gallates showed no significant inhibition at 100 μM. Similarly, r3β-HSD4 displayed a comparable inhibition pattern but was more sensitive, with IC50 values of 49.60, 31.86, 25.14, 10.79, and 5.91 μM for the same gallates, respectively. Bivariate correlation analysis demonstrated a negative correlation between IC50 values and hydrophobicity, molecular weight, heavy atom number, carbon number, rotatable bonds, fraction of sp3-hybridized carbon atoms, and volume, while a positive correlation was observed with the lowest binding energy. Molecular docking analysis indicated that these gallates bind to cofactor binding sites or between the steroid and cofactor binding sites. Additionally, 3D-QSAR modeling highlighted the influence of hydrogen bond acceptors and hydrophobic regions on the inhibitory activity of these compounds. The suppression of progesterone production by these gallates in intact JAr cells is influenced not only by their inhibitory potency on h3β-HSD1 but also by their aqueous solubility. These findings provide valuable insights into the endocrine-disrupting potential of gallates and their interaction with 3β-HSDs.
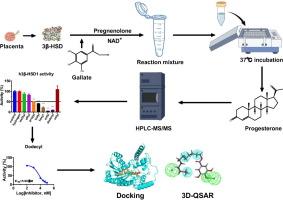
没食子酸酯对人和大鼠胎盘3β-羟基类固醇脱氢酶的抑制作用:联合实验、3D-QSAR和硅分析
没食子酸盐被广泛用作食品、药品和化妆品的添加剂。尽管它们被广泛使用,但它们对内分泌系统的影响仍然知之甚少。本研究探讨了没食子酸酯与3β-羟基类固醇脱氢酶(3β-HSD)的潜在相互作用,3β-HSD是胎盘黄体酮合成的关键酶。研究了9种没食子酸酯对人(h3β-HSD1)和大鼠(r3β-HSD4)胎盘酶的抑制作用、构效关系(SAR)和3D-QSAR。结果表明,没食子酸酯对h3β-HSD1的抑制作用取决于醇部分的碳链长度。没食子酸丙酯、丁基、己基、辛酯和十二基没食子酸酯表现出混合抑制作用,IC50值分别为75.16、64.59、25.44、19.56和5.99 μM,而没食子酸、甲基、乙基和没食子酸十六酯在100 μM范围内无显著抑制作用。同样,r3β-HSD4表现出类似的抑制模式,但更敏感,对相同的没食子酸盐的IC50值分别为49.60,31.86,25.14,10.79和5.91 μM。双变量相关分析表明,IC50值与疏水性、分子量、重原子序数、碳数、可旋转键、sp3杂化碳原子分数、体积呈负相关,与最低结合能呈正相关。分子对接分析表明,这些没食子酸酯结合在辅因子结合位点或类固醇与辅因子结合位点之间。此外,3D-QSAR模型强调了氢键受体和疏水区域对这些化合物抑制活性的影响。这些没食子酸酯在完整JAr细胞中抑制孕酮的产生不仅受其对h3β-HSD1的抑制效力的影响,还受其水溶性的影响。这些发现为没食子酸盐的内分泌干扰潜力及其与3β- hsd的相互作用提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


