Theoretical study on the effect of H2O on the formation mechanism of NOx precursor during 2-pyrrolidone pyrolysis
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The nitrogen in the pyrolysis process of kitchen waste partially originates from proteins (amino acids), and 2-pyrrolidone is a product with high content of glutamic acid. Density functional theory (DFT) was used to study the effect of H2O on NOx precursors (NH3, HCN, HNCO) produced by the pyrolysis of 2-pyrrolidone. Compared with the reaction without H2O, the reaction path and the results of the product have changed in varying degrees. This study found that NH3 is more prone to form than HCN, which was consistent with the phenomenon that the release of NH3 was higher than that of HCN during pyrolysis. In addition, it was found that H2O reduced the reaction energy barrier of NH3 and HCN during the pyrolysis of 2-pyrrolidone, and promoted the formation of NH3 and HCN.
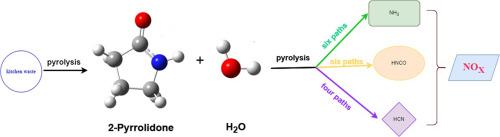
2-吡咯烷酮热解过程中H2O对NOx前驱体形成机理影响的理论研究
餐厨垃圾热解过程中的氮部分来源于蛋白质(氨基酸),2-吡咯烷酮是谷氨酸含量较高的产物。采用密度泛函理论(DFT)研究了H2O对2-吡罗烷酮热解生成的NOx前驱体NH3、HCN、HNCO的影响。与无水反应相比,反应路径和产物结果都有不同程度的变化。本研究发现NH3比HCN更容易形成,这与热解过程中NH3的释放量高于HCN的现象一致。此外,还发现H2O降低了2-吡咯烷酮热解过程中NH3和HCN的反应能垒,促进了NH3和HCN的生成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


