Investigation of the binding affinity of a newly synthesized copper(II) complex to DNA and enzymes (catalase/trypsin/urease) using spectrofluorimetry and in silico approaches
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In recent years, there has been a growing interest in the synthesis of novel compounds with medicinal potential, particularly those exhibiting antioxidant properties, due to their ability to delay, prevent, or eliminate oxidative damage in target cells. Understanding the interactions between these compounds and major biological targets—including DNA, catalase, trypsin, and urease—is essential for improving their bioactivity and therapeutic potential. The synthesis and comprehensive characterization of a novel copper(II) complex, [Cu(3,5ClSal-Phe)(CH₃OH)]—featuring a Schiff base ligand derived from 3,5-chlorosalicylaldehyde and L-phenylalanine—were carried out using electronic absorption spectroscopy, FTIR, ESI-MS, ESR and X-ray diffraction. Electronic absorption and fluorescence spectroscopy were employed to investigate the interactions between the complex and key biomolecules such as CT-DNA, catalase, trypsin, and urease. The complex was found to bind CT-DNA through minor groove interaction, while its fluorescence quenching with catalase, trypsin, and urease proceeds via a static mechanism. To better understand the molecular basis of these biological effects, docking simulations were employed using DNA and three key enzymes, trypsin, urease, and catalase, as molecular targets. Among all targets, the strongest binding affinity was observed with catalase (−9.31 kcal/mol), where the complex formed hydrogen bonds with Arg111, His361, Phe333, and Arg71, as well as a halogen interaction with Tyr357. Interactions with trypsin and urease were also energetically favorable, predominantly involving polar and hydrophobic residues. Docking protocols were validated through redocking (RMSD <2.0 Å), ensuring reliability of the predicted binding modes. In vitro assessment of the complex's antioxidant activity, conducted using the DPPH radical scavenging assay, revealed a moderate scavenging efficiency.
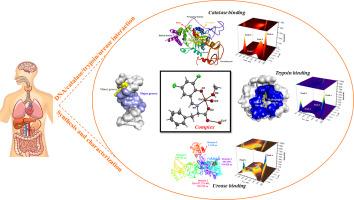
新合成的铜(II)配合物对DNA和酶(过氧化氢酶/胰蛋白酶/脲酶)的结合亲和力的荧光光谱和硅法研究
近年来,人们对合成具有药用潜力的新化合物越来越感兴趣,特别是那些具有抗氧化特性的化合物,因为它们能够延缓、预防或消除靶细胞的氧化损伤。了解这些化合物与主要生物靶点(包括DNA、过氧化氢酶、胰蛋白酶和脲酶)之间的相互作用,对于提高它们的生物活性和治疗潜力至关重要。采用电子吸收光谱、FTIR、ESI-MS、ESR和x射线衍射等手段合成了以3,5-氯水杨醛和l-苯丙氨酸为配体的新型铜(II)配合物[Cu(3,5 clsal -phe)(CH₃OH)]并对其进行了综合表征。利用电子吸收和荧光光谱研究了复合物与CT-DNA、过氧化氢酶、胰蛋白酶和脲酶等关键生物分子的相互作用。该复合物通过轻微的凹槽相互作用与CT-DNA结合,而其与过氧化氢酶、胰蛋白酶和脲酶的荧光猝灭则通过静态机制进行。为了更好地理解这些生物效应的分子基础,以DNA和三种关键酶(胰蛋白酶、脲酶和过氧化氢酶)作为分子靶点进行对接模拟。其中,与过氧化氢酶的结合亲和力最强(- 9.31 kcal/mol),与Arg111、His361、Phe333和Arg71形成氢键,并与Tyr357发生卤素相互作用。与胰蛋白酶和脲酶的相互作用在能量上也是有利的,主要涉及极性和疏水残基。通过重新对接(RMSD <2.0 Å)验证对接协议,确保了预测绑定模式的可靠性。体外评估的配合物的抗氧化活性,进行了使用DPPH自由基清除试验,显示中等清除效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


