A Convergent Approach to Phragmalin-Type Limonoids: Total Synthesis of Thaigranatin P
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Herein, we report the total synthesis of erythrocarpines M and L and thaigranatin P, topologically complex phragmalin-type limonoids, the latter exhibiting potent agonism on the human pregnane X receptor (hPXR). A key transformation is a SmI2-mediated reductive coupling of an aldehyde and an alkene, which constructs the signature tricyclo[3.3.12,10.11,4]decane framework characteristic of phragmalin natural products. The synthesis features a strategically convergent construction of the phragmalin core structure via a cuprate addition, triflation, and intramolecular Heck reaction sequence. This route offers a versatile platform for the synthesis of structurally diverse phragmalin analogs for future biological investigation.
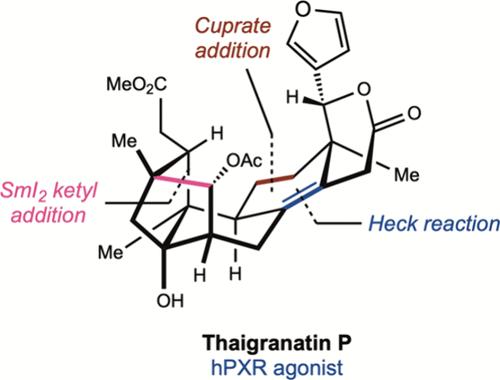
一种聚类合成phragmalin型类柠檬素的方法:thigranatin P的全合成
在此,我们报道了红卡松素M和L和苔格兰素P的全合成,这是拓扑结构复杂的phragmalin型柠檬素,后者对人妊娠X受体(hPXR)具有强烈的激动作用。一个关键的转化是smi2介导的醛和烯烃的还原偶联,构建了phragmalin天然产物特征的三环[3.3.12,10.11,4]癸烷框架。该合成的特点是通过适当的加成,膨胀和分子内Heck反应序列战略性地收敛了phragmalin核心结构的构建。该途径为合成结构多样的phragmalin类似物提供了一个多功能的平台,为未来的生物学研究提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


