Metabolomic profiling reveals ADB-BUTINACA-induced long-term hepatotoxicity
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The multi-organ toxicity of ADB-BUTINACA, a synthetic cannabinoid with high abuse potential, poses a public health risk. However, its long-term toxicological effects and metabolic mechanisms remain unclear. This study aimed to elucidate the hepatotoxic potential and metabolic disruption induced by ADB-BUTINACA through a 30-day oral exposure model in mice at doses of 0.1, 1, and 10 mg kg−1·bw−1. Serum biochemical analysis revealed elevated TNF-α, IL-6, total cholesterol, triglycerides, and LDL-C, indicating systemic inflammation and lipid dysregulation. Histopathological examination showed dose-dependent hepatocellular swelling, lipid droplet accumulation, and inflammatory infiltration. Non-targeted metabolomic profiling identified 60 significantly altered metabolites and 12 perturbed metabolic pathways, including unsaturated fatty acid biosynthesis, taurine metabolism, and the tricarboxylic acid (TCA) cycle. Notably, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and taurine were markedly reduced. RT-qPCR results showed that ADB-BUTINACA induced inhibition of TCA cycle and unsaturated fatty acid synthesis activity in the liver. Molecular docking demonstrated stable and competitive binding of ADB-BUTINACA to key metabolic enzymes: Fatty Acid Desaturase 2 (FADS2) and Elongation of Very Long Chain Fatty Acids Protein 2 (ELOVL2). This suggests interference with endogenous lipid regulatory processes. These findings elucidate the hepatotoxic mechanisms of ADB-BUTINACA and underscore its potential health risks as an emerging synthetic cannabinoid contaminant.
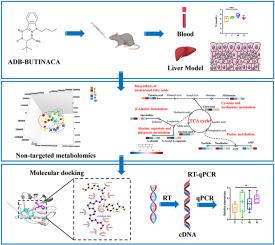
代谢组学分析显示adb - butinaca诱导的长期肝毒性。
ADB-BUTINACA是一种具有高度滥用潜力的合成大麻素,其多器官毒性对公众健康构成威胁。然而,其长期毒理学效应和代谢机制尚不清楚。本研究旨在通过小鼠口服0.1、1和10 mg·kg-1·bw-1剂量的ADB-BUTINACA 30天暴露模型,阐明ADB-BUTINACA的肝毒性潜力和代谢破坏。血清生化分析显示TNF-α、IL-6、总胆固醇、甘油三酯和LDL-C升高,提示全身性炎症和脂质失调。组织病理学检查显示剂量依赖性肝细胞肿胀,脂滴积聚,炎症浸润。非靶向代谢组学分析鉴定了60种显著改变的代谢物和12种代谢途径,包括不饱和脂肪酸生物合成、牛磺酸代谢和三羧酸(TCA)循环。值得注意的是,二十碳五烯酸(EPA)、二十二碳六烯酸(DHA)和牛磺酸明显减少。RT-qPCR结果显示,ADB-BUTINACA诱导肝脏TCA循环和不饱和脂肪酸合成活性的抑制。分子对接证明了ADB-BUTINACA与关键代谢酶:脂肪酸去饱和酶2 (FADS2)和超长链脂肪酸延伸蛋白2 (ELOVL2)的稳定和竞争性结合。这表明内源性脂质调节过程受到干扰。这些发现阐明了ADB-BUTINACA的肝毒性机制,并强调了其作为一种新兴的合成大麻素污染物的潜在健康风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


