Inhibiting MARS attenuates hyperhomocysteinemia-associated neurodegeneration in Parkinson's disease
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Hyperhomocysteinemia is an independent risk factor for Parkinson's disease (PD). Homocysteine (Hcy) is converted to Hcy thiolactone (HTL) in error-editing reactions catalyzed by methionine-tRNA synthetase (MARS). HTL forms isopeptide bonds with lysine residues of target proteins in a process known as N-homocysteinylation (N-Hcy), which contributes to the neurotoxicity of Hcy in PD pathogenesis. Thus, MARS may represent a potential target for controlling hyperhomocysteinemia-associated neurotoxicity. Here we tested the effect of MARS on protein N-Hcy in cultured cells, MPTP-induced mouse model of PD, and mice injected with α-synuclein PFFs. We found that the protein N-Hcy levels were increased in the brains of PD model mice. MARS knockdown ameliorates protein N-Hcy, oxidative stress, mitochondrial dysfunction, and α-synuclein aggregation in cells. MARS knockdown also alleviated dopaminergic neurodegeneration and behavioral deficits in mice injected with MPTP. In mice injected with α-synuclein fibrils, MARS knockdown attenuated α-synuclein aggregation, dopaminergic neurodegeneration, and motor impairments. These results indicate that inhibition of MARS attenuates PD-like pathology induced by hyperhomocysteinemia.
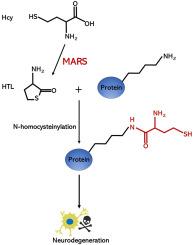
抑制MARS可减轻帕金森病中高同型半胱氨酸血症相关的神经变性
高同型半胱氨酸血症是帕金森病(PD)的独立危险因素。在蛋氨酸- trna合成酶(MARS)催化的错误编辑反应中,同型半胱氨酸(Hcy)转化为Hcy硫内酯(HTL)。HTL与靶蛋白的赖氨酸残基形成异肽键,这一过程被称为n -同型半胱氨酸化(N-Hcy),这有助于Hcy在PD发病机制中的神经毒性。因此,MARS可能是控制高同型半胱氨酸血症相关神经毒性的潜在靶点。我们在培养细胞、mptp诱导的PD小鼠模型和注射α-突触核蛋白pff小鼠中检测了MARS对蛋白N-Hcy的影响。我们发现PD模型小鼠脑内蛋白N-Hcy水平升高。MARS敲低可改善细胞中蛋白N-Hcy、氧化应激、线粒体功能障碍和α-突触核蛋白聚集。MARS敲低也能减轻注射MPTP小鼠的多巴胺能神经变性和行为缺陷。在注射α-突触核蛋白原纤维的小鼠中,MARS抑制α-突触核蛋白聚集、多巴胺能神经变性和运动损伤。这些结果表明,抑制MARS可减轻高同型半胱氨酸血症引起的pd样病理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


