Pro-inflammatory and oxidative responses to burn pit relevant desert particulate matter in macrophages: A role for TLR2 signaling
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Particulate matter (PM) exposure is linked to many respiratory diseases such as asthma, yet the underlying mechanisms remain unclear. A large number of warfighters deployed to Afghanistan and Iraqi have developed deployment related respiratory disease. This study investigates the effects of burn pit-relevant desert PM from Afghanistan (APM) and comparative desert PM from China Lake, California (CPM) on oxidative stress and pro-inflammatory responses in macrophages. Using mouse monocyte cell line and primary bone marrow-derived macrophages (BMDMs) with or without Toll-like Receptor 2 (TLR2) deficiency, we assessed nitric oxide (NO.), hydrogen peroxide (H2O2), and cytokine production using biochemical assays, enzyme-linked immunosorbent assay (ELISA), pharmacological and genetic modulation, and bulk RNA sequencing. APM was more cytotoxic than CPM in monocytes. Both PMs increased H2O2 levels, with acellular conditions generating higher H2O2 levels, which was catalase-sensitive and attenuated by metal chelation. APM induced stronger NO. and cytokine C-X-C motif chemokine ligand 1 (CXCL1) responses than CPM, with NO. production attenuated by the nitric oxide synthase (NOS) inhibitor L-NG-nitroarginine methyl ester (LNAME), which also prevented cytotoxicity. TLR2 activation via agonist Pam3CSK4 enhanced NO. and CXCL1, while inhibition with antagonist C29 or TLR2 knockout partially suppressed APM-induced responses. Bulk RNA sequencing revealed that APM upregulated M1 pro-inflammatory polarization markers, many of which were significantly reduced in TLR2-deficient BMDMs. These findings demonstrate that APM exposure of monocytes/macrophages elicits stronger oxidative and pro-inflammatory responses than CPM, partially mediated through TLR2 signaling. This study provides mechanistic insight into deployment-related PM exposure and identifies potential therapeutic targets to mitigate inflammation in affected individuals.
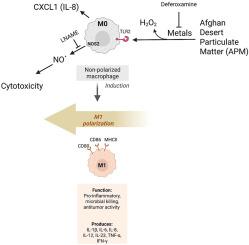
巨噬细胞对烧伤坑相关沙漠颗粒物质的促炎和氧化反应:TLR2信号的作用
颗粒物(PM)暴露与哮喘等许多呼吸系统疾病有关,但其潜在机制尚不清楚。部署到阿富汗和伊拉克的大量作战人员患上了与部署有关的呼吸道疾病。本研究探讨了来自阿富汗的烧伤坑相关沙漠PM (APM)和来自加利福尼亚中国湖的比较沙漠PM (CPM)对巨噬细胞氧化应激和促炎反应的影响。使用小鼠单核细胞系和原代骨髓源性巨噬细胞(bmmdms),有或没有toll样受体2 (TLR2)缺陷,我们使用生化测定、酶联免疫吸附测定(ELISA)、药理学和遗传调节以及大量RNA测序来评估一氧化氮(NO)、过氧化氢(H2O2)和细胞因子的产生。在单核细胞中,APM比CPM更有细胞毒性。两种pm都增加了H2O2水平,在非细胞条件下产生更高的H2O2水平,过氧化氢酶对其敏感,并通过金属螯合作用减弱。APM诱导NO增强。细胞因子C-X-C基序趋化因子配体1 (CXCL1)的反应较CPM明显。一氧化氮合酶(NOS)抑制剂l - ng -硝基精氨酸甲酯(LNAME)也能抑制细胞毒性。通过激动剂Pam3CSK4激活TLR2增强NO。而拮抗剂C29或TLR2敲除抑制部分抑制了apm诱导的反应。大量RNA测序显示,APM上调了M1促炎极化标记物,其中许多标记物在tlr2缺陷bmdm中显著降低。这些发现表明,APM暴露于单核细胞/巨噬细胞比CPM引发更强的氧化和促炎反应,部分通过TLR2信号介导。该研究为部署相关的PM暴露提供了机制见解,并确定了减轻受影响个体炎症的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


