Targeting FDX1 with Icaritin attenuates neuronal cuproptosis by reconciling mitochondrial fission-fusion dynamics and bioenergetic homeostasis
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Copper overload triggers cuproptosis, a copper-dependent cell death pathway characterized by mitochondrial oxidative stress, dysfunction, and disrupted dynamics, posing significant threats to neuronal health. Icaritin (ICT), a bioactive flavonoid from Herbal Epimedii, exhibits antioxidant and neuroprotective properties, but its impact on cuproptosis remains unexplored. Thus, this study was aimed to investigate ICT's protective mechanisms against cuproptosis induced by the cupric sulfate and copper ionophore elesclomol (Cu-ES) in HT22 hippocampal neuronal cells. We found that Cu-ES effectively modeled cuproptosis, reducing viability by 50 % and inducing severe mitochondrial damage, oxidative stress, dysfunction, tricarboxylic acid cycle disruption, and dynamics imbalance. While ICT's treatment concentration-dependently mitigated these injuries. Mechanistically, computational molecular interaction analysis and trajectory simulations, and surface plasmon resonance confirmed ICT directly binds ferredoxin 1 (FDX1) with high affinity and stability, downregulating its protein expression. ICT consequently inhibited the FDX1-mediated cuproptosis pathway, reducing dihydrolipoamide S-acetyltransferase (DLAT) oligomerization, modulating cuproptosis sensitivity proteins, restoring copper homeostasis by increasing ATPase copper transporting beta (ATP7B) and decreasing solute carrier family 31 member 1 (SLC31A1), and suppressing the lipoylation pathway. Crucially, FDX1 knockdown abolished Cu-ES toxicity and potentiated ICT's protective effects against superoxide production, DLAT expression, and copper accumulation. Furthermore, ICT rescued mitochondrial dynamics by promoting fusion and inhibiting fission. Our findings demonstrate ICT is a potent inhibitor of neuronal cuproptosis, targeting FDX1 to alleviate mitochondrial oxidative stress, dysfunction, and dynamics disorder, presenting a promising therapeutic strategy.
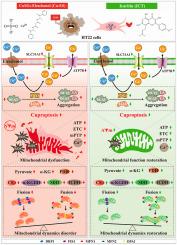
以FDX1为靶点,用淫羊藿苷调节线粒体分裂融合动力学和生物能量稳态,从而减弱神经元铜突起。
铜过载触发铜细胞凋亡,这是一种依赖铜的细胞死亡途径,以线粒体氧化应激、功能障碍和动力学破坏为特征,对神经元健康构成重大威胁。淫羊藿苷(Icaritin, ICT)是一种从淫羊藿中提取的具有生物活性的类黄酮,具有抗氧化和神经保护作用,但其对铜变形的影响尚不清楚。因此,本研究旨在探讨ICT对硫酸铜和铜离子载体埃雷斯克莫尔(Cu-ES)诱导的HT22海马神经元细胞铜退化的保护机制。我们发现Cu-ES有效地模拟了铜残症,使存活能力降低50%,并诱导严重的线粒体损伤、氧化应激、功能障碍、三羧酸循环中断和动力学失衡。而ICT的治疗浓度依赖性减轻了这些损伤。机制上,计算分子相互作用分析和轨迹模拟以及表面等离子体共振证实ICT以高亲和力和稳定性直接结合铁氧还蛋白1 (FDX1),下调其蛋白表达。因此,ICT抑制了fdx1介导的铜化途径,减少了二氢脂酰胺s -乙酰转移酶(DLAT)寡聚,调节了铜化敏感蛋白,通过增加ATPase铜转运β (ATP7B)和减少溶质载体家族31成员1 (SLC31A1)来恢复铜稳态,并抑制了脂化途径。重要的是,FDX1基因敲除消除了Cu-ES毒性,增强了ICT对超氧化物产生、DLAT表达和铜积累的保护作用。此外,ICT通过促进融合和抑制裂变来挽救线粒体动力学。我们的研究结果表明ICT是一种有效的神经元铜突抑制剂,靶向FDX1缓解线粒体氧化应激、功能障碍和动力学障碍,呈现出一种很有前景的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


