Kinetics of Sulfuric Acid Decomposition of Nepheline
IF 0.6
4区 工程技术
Q4 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
A mathematical analysis of the obtained experimental data was performed using topochemical equations in a linearized form: Kazeev–Erofeev–Kolmogorov, Ginstling–Brounshtein, Jander, and Gray–Weddington equations. It was found that the interaction mechanism of nepheline concentrate with sulfuric acid is most accurately described by the Ginstling–Brounshtein equation. The activation energy of the process was determined, based on which it was concluded that the process is controlled by kinetics.
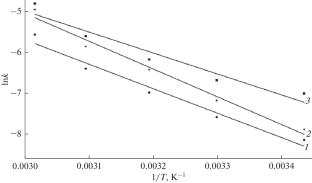
霞石硫酸分解动力学研究
利用线性化形式的拓扑化学方程:Kazeev-Erofeev-Kolmogorov, ginstling - bronshtein, Jander和Gray-Weddington方程对获得的实验数据进行数学分析。研究发现,用ginstling - browshtein方程最准确地描述霞石精矿与硫酸的相互作用机理。测定了反应的活化能,得出反应受动力学控制的结论。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.20
自引率
25.00%
发文量
70
审稿时长
24 months
期刊介绍:
Theoretical Foundations of Chemical Engineering is a comprehensive journal covering all aspects of theoretical and applied research in chemical engineering, including transport phenomena; surface phenomena; processes of mixture separation; theory and methods of chemical reactor design; combined processes and multifunctional reactors; hydromechanic, thermal, diffusion, and chemical processes and apparatus, membrane processes and reactors; biotechnology; dispersed systems; nanotechnologies; process intensification; information modeling and analysis; energy- and resource-saving processes; environmentally clean processes and technologies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


