Synthesis and Neurotropic Activity of Cyclic and Spirocyclic Derivatives of 1,3-Diazadamantane
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Cyclic and spirocyclic 1,3-diazaadamantane derivatives were synthesized by the condensation of substituted 3,7-diazabicyclo[3.3.1]nonanes with cyclic and heterocyclic aldehydes and ketones. Screening for neurotropic activity showed that spirocyclic 1,3-diazaadamantanes exhibit a higher potency than their cyclic analogs, with activity levels of 40–60 against 20–40%, respectively.
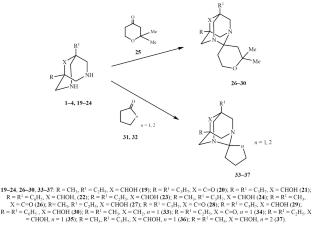
1,3-重氮金刚烷环和螺环衍生物的合成及其嗜神经活性
以取代的3,7-重氮杂环壬烷[3.3.1]与环、杂环醛和酮缩合为原料,合成了环和螺环1,3-重氮杂烷衍生物。对促神经活性的筛选表明,螺环1,3-重氮adamantanes比其环类似物表现出更高的效力,活性水平分别为40 - 60%和20-40%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


