Single-cell resolution spatial analysis of antigen-presenting cancer-associated fibroblast niches
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Recent studies identify a unique subtype of cancer-associated fibroblasts (CAFs) termed antigen-presenting CAFs (apCAFs), which remain poorly understood. To gain a comprehensive understanding of the origin and function of apCAFs, we construct a fibroblast molecular atlas across 15 types of tissues and solid tumors. Our integration study unexpectedly reveals two distinct apCAF populations present in most cancer types: one associated with mesothelial-like cells and the other with fibrocytes. Using a high-resolution single-cell spatial imaging platform, we characterize the spatial niches of these apCAF populations. We find that mesothelial-like apCAFs are located near cancer cells, while fibrocyte-like apCAFs are associated with lymphocyte-enriched niches. Additionally, we discovered that both apCAF populations can up-regulate secreted phosphoprotein 1 (SPP1), which facilitates primary tumor formation, peritoneal metastasis, and therapy resistance. Taken together, this study offers an unprecedented resolution in analyzing apCAFs and their spatial niches.
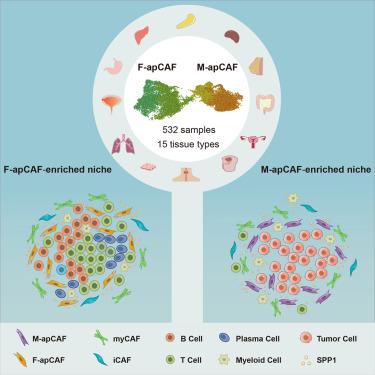
抗原呈递癌相关成纤维细胞龛的单细胞分辨率空间分析
最近的研究发现了一种独特的癌症相关成纤维细胞(CAFs)亚型,称为抗原呈递CAFs (apCAFs),但对其知之甚少。为了全面了解apCAFs的起源和功能,我们构建了横跨15种组织和实体肿瘤的成纤维细胞分子图谱。我们的整合研究意外地揭示了两种不同的apCAF群体存在于大多数癌症类型中:一种与间皮样细胞相关,另一种与纤维细胞相关。利用高分辨率单细胞空间成像平台,我们描述了这些apCAF种群的空间生态位。我们发现间皮样apCAFs位于癌细胞附近,而纤维细胞样apCAFs与淋巴细胞富集的生态位相关。此外,我们发现这两个apCAF群体都可以上调分泌的磷酸化蛋白1 (SPP1),这促进了原发性肿瘤的形成、腹膜转移和治疗抵抗。综上所述,本研究为分析apCAFs及其空间生态位提供了前所未有的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


