Ru-Diphosphine catalyzed asymmetric hydrogenation of unprotected β-C-glycosidic ketones
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Stereoselective reduction of β-C-glycosidic ketones is an effective method for the synthesis of carbohydrate derivatives. We herein report that β-C-glycosidic ketones, not generally considered as functionalized ketones, are unexpectedly hydrogenated by Ru-diphosphine catalysts with excellent stereoselectivity (up to >99 : 1 dr). Further investigation into the effect of the chiral hydroxyl groups during the reaction has also been conducted. This reaction has been performed on a multi-gram scale, providing a novel approach for the construction of chiral centers in carbohydrate derivatives.
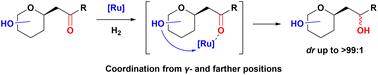
钌二膦催化无保护β- c -糖苷酮的不对称加氢反应
立体选择还原β- c -糖苷酮是合成碳水化合物衍生物的有效方法。我们在此报道,通常不被认为是功能化酮的β- c -糖苷酮,意外地被具有优异立体选择性(高达>;99: 1 dr)的ru -二膦催化剂氢化。对手性羟基在反应中的作用也进行了进一步的研究。该反应在多克尺度上进行,为碳水化合物衍生物的手性中心的构建提供了一种新的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


