Entropy Governs the Structure and Reactivity of Water Dissociation Under Electric Fields
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The response of water to electric fields is critical to the performance and stability of electrochemical devices, and the selectivity of enzymatic, atmospheric, and organic reactions. A key process in this context is the water (auto)dissociation reaction (WD), which governs acid–base aqueous chemistry and shapes reaction rates and mechanisms. Despite its significance, the thermodynamics of the WD reaction in electrified environments remains poorly understood. Here, we investigate the WD reaction under external electric fields using ab initio molecular dynamics simulations within the framework of the modern theory of polarization. Our results reveal that strong electric fields dramatically enhance the WD reaction, increasing the equilibrium constant by several orders of magnitude. Moreover, we show that the applied field transforms the WD reaction from an entropically hindered process to an entropy-driven one. Analysis shows that this is because the electric field alters the tendency of ions to be structure makers or structure breakers. By highlighting how strong electric fields reshape solvent organization and reactivity, this work opens new avenues for designing aqueous electro-catalysts that leverage solvent entropy to enhance their performance.
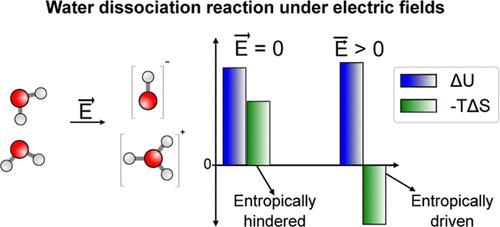
电场作用下水解离的结构和反应性由熵决定
水对电场的响应对电化学装置的性能和稳定性以及酶、大气和有机反应的选择性至关重要。在这种情况下,一个关键的过程是水(自动)解离反应(WD),它决定了酸碱水化学,并决定了反应速率和机制。尽管具有重要意义,但通电环境下WD反应的热力学仍然知之甚少。本文在现代极化理论的框架下,利用从头算分子动力学模拟研究了外电场下的WD反应。我们的研究结果表明,强电场显著增强了WD反应,使平衡常数提高了几个数量级。此外,我们还证明了外加电场将WD反应从熵阻碍过程转变为熵驱动过程。分析表明,这是因为电场改变了离子成为结构制造者或结构破坏者的倾向。通过强调强电场如何重塑溶剂组织和反应性,这项工作为设计利用溶剂熵来提高其性能的水性电催化剂开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


