Use of Electron-Transfer Mediators to Access Higher Electrochemical Current Densities in Ni-Catalyzed Cross-Electrophile Coupling
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Ni-catalyzed cross-electrophile coupling (XEC) reactions are versatile methods for carbon–carbon bond formation, and they often use electrochemistry to supply the requisite electrons. These reactions, however, typically exhibit slow rates and operate at low current densities (e.g., 1–4 mA/cm2) to avoid formation of undesirable side products. Here, we show that much higher current densities and reaction selectivity can be accessed by using a homogeneous electron-transfer (ET) mediator. Bis(ethylcyclopentadienyl)cobalt(II), which has a redox potential similar to that of the bipyridyl-ligated Ni catalyst (−1.45 and –1.50 V vs Fc/Fc+, respectively), exhibits the best performance. This ET-mediator strategy is applied to numerous C(sp2)–C(sp3) coupling reactions and extended to a flow-based reaction on 10 mmol-scale that proceeds in 96% yield with 91% Faradaic efficiency at a current density of 18 mA/cm2.
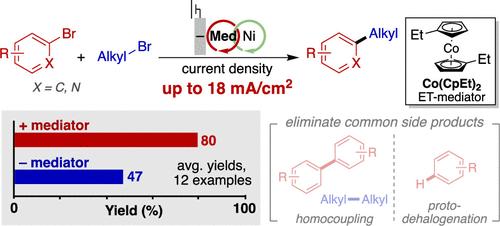
利用电子转移介质在镍催化的亲电偶联中获得更高的电化学电流密度
镍催化的交叉亲电偶联反应(XEC)是碳-碳键形成的通用方法,它们通常使用电化学来提供必要的电子。然而,这些反应通常表现出缓慢的速率,并在低电流密度(例如,1-4毫安/平方厘米)下进行,以避免形成不希望的副产物。在这里,我们表明可以通过使用均匀电子转移(ET)介质获得更高的电流密度和反应选择性。双(乙基环戊二烯基)钴(II)具有与联吡啶连接镍催化剂相似的氧化还原电位(分别为- 1.45和-1.50 V vs Fc/Fc+),表现出最好的性能。这种et介体策略应用于众多C(sp2) -C (sp3)偶联反应,并扩展到10 mmol尺度的流动反应,在18 mA/cm2的电流密度下,产率为96%,法拉第效率为91%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


