Targeting cuproptosis by FDX1 in acetaminophen-induced liver injury
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Acetaminophen (APAP) overdose is the most common etiology of acute drug-induced liver injury (DILI) worldwide. Although oxidative stress, apoptosis, and necrosis are well-recognised mechanisms, the involvement of cuproptosis, a newly identified, copper-dependent form of regulated cell death, remains poorly understood. Here, the contribution of cuproptosis to APAP hepatotoxicity is elucidated, identifying ferredoxin 1 (FDX1) as its master regulator. In vivo, the copper chelator ammonium tetrathiomolybdate (TM) markedly attenuated APAP-induced liver damage, as evidenced by decreased serum ALT and AST levels, reduced hepatocyte death, and diminished inflammatory cell infiltration and cytokine release. Transcriptomic mining of GEO datasets revealed significant dysregulation of cuproptosis-associated genes in APAP-exposed hepatocytes, with FDX1 being the most prominently up-regulated. Genetic ablation of FDX1 in mice and FDX1 knockdown in human HepaRG cells both conferred robust protection against APAP-induced lethality, phenocopying the effects of TM. Collectively, our findings establish FDX1-mediated cuproptosis as a critical driver of APAP hepatotoxicity and position copper homeostasis as a tractable therapeutic target for DILI.
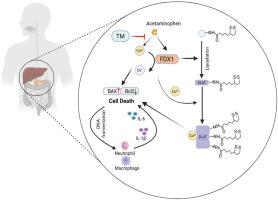
FDX1在对乙酰氨基酚诱导的肝损伤中的靶向性cuprotosis。
对乙酰氨基酚(APAP)过量是世界范围内急性药物性肝损伤(DILI)最常见的病因。虽然氧化应激、细胞凋亡和坏死是众所周知的机制,但铜增生(一种新发现的依赖铜的调节细胞死亡形式)的参与仍然知之甚少。本研究阐明了铜中毒对APAP肝毒性的贡献,确定了铁氧还蛋白1 (FDX1)是其主要调节因子。在体内,铜螯合剂四硫钼酸铵(TM)显著减轻apap诱导的肝损伤,表现为降低血清ALT和AST水平,减少肝细胞死亡,减少炎症细胞浸润和细胞因子释放。GEO数据集的转录组学挖掘显示,apap暴露的肝细胞中铜裂相关基因显著失调,其中FDX1的上调最为显著。小鼠FDX1基因消融和人类HepaRG细胞中FDX1基因敲低均具有强大的保护作用,可抵抗apap诱导的致死性,表型复制TM的作用。总的来说,我们的研究结果证实了fdx1介导的铜沉积是APAP肝毒性的关键驱动因素,并将铜稳态定位为DILI的一个可处理的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


