Chemoproteomics-based profiling reveals the targeting molecular mechanism of reversal of cisplatin resistance by baicalin in head and neck squamous cell carcinoma
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Cisplatin resistance is a major cause of chemotherapy failure and poor prognosis in patients with head and neck squamous cell carcinoma (HNSCC). The combination of natural compounds and platinum-based agents can overcome cisplatin resistance. Here, we demonstrate that baicalin, one of the major active flavonoids extracted from the root of the medicinal herb Scutellaria baicalensis Georgi, reverses cisplatin resistance in HNSCC in vitro and in vivo. Mechanistically, glutathione S-transferase (GST)-π subtype, also known as GSTP1, was identified as a direct target of baicalin, and the cysteine 47 (cys47) of GSTP1 is modified by baicalin, which is mediated by the Michael reaction. Moreover, baicalin increased the accumulation of cisplatin and activated the c-Jun N-terminal kinase (JNK) pathway in wild-type cisplatin-resistant HNSCC cells, but its effects were lost in cys47-mutated cisplatin-resistant HNSCC cells. Meanwhile, cys47 mutation of GSTP1 abrogated baicalin-induced reversal of cisplatin resistance in HNSCC cells. In conclusion, we demonstrate a novel mechanism for the reversal of cisplatin resistance by baicalin, confirming that baicalin can inhibit GSTP1 activity by interacting with the Cys47 residue of GSTP1, thereby enhancing intracellular accumulation of cisplatin or activation of the JNK pathway. This study lays the foundation for the use of baicalin to overcome cisplatin resistance in HNSCC.
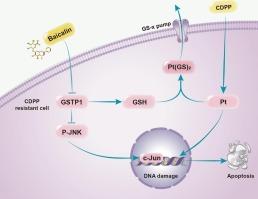
基于化学蛋白质组学的分析揭示了黄芩苷逆转头颈部鳞状细胞癌顺铂耐药的靶向分子机制。
顺铂耐药是头颈部鳞状细胞癌(HNSCC)患者化疗失败和预后不良的主要原因。天然化合物与铂基药物联合使用可克服顺铂耐药。本研究证明黄芩苷是黄芩根中提取的主要活性黄酮类化合物之一,在体内和体外均可逆转HNSCC的顺铂耐药。机制上,谷胱甘肽s -转移酶(GST)-π亚型,也称为GSTP1,被确定为黄芩苷的直接靶标,GSTP1的半胱氨酸47 (cys47)被黄芩苷修饰,并通过Michael反应介导。此外,在野生型顺铂耐药HNSCC细胞中,黄芩苷增加了顺铂的积累,激活了c-Jun n-末端激酶(JNK)通路,但在cys47突变的顺铂耐药HNSCC细胞中,黄芩苷的作用消失。同时,GSTP1的cys47突变取消了黄芩苷诱导的HNSCC细胞顺铂耐药的逆转。总之,我们展示了黄芩苷逆转顺铂耐药的新机制,证实黄芩苷可以通过与GSTP1的Cys47残基相互作用抑制GSTP1的活性,从而增强顺铂在细胞内的积累或激活JNK通路。本研究为黄芩苷克服HNSCC顺铂耐药奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


