ETV5 transcriptionally inhibits KIF23 to repress pyroptosis in aged mice with perioperative neurocognitive disorders
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Caspase-3/gasdermin E (GSDME)-dependent pyroptosis activation has emerged as a potential mechanism contributing to perioperative neurocognitive disorders (PND). Public transcriptome profiling identified the diminution of the transcription factor E-twenty-six variant 5 (ETV5) in the hippocampus of aged PND mice. This study intended to investigate the role of ETV5 and its target genes in the pathogenesis of PND. Bioinformatics analysis identified kinesin family member 23 (KIF23) as a putative target gene of ETV5. The PND mouse model was established via laparotomy under isoflurane anesthesia after treatment with recombinant adeno-associated virus 9 (AAV9) to overexpress ETV5 and/or KIF23. HT22 neurons were transfected with either pcDNA3.1-ETV5 or siETV5, followed by treatment with isoflurane and lipopolysaccharide (Iso + LPS). Cognitive behavior, TUNEL staining, and pyroptosis-associated indicators were assessed. ETV5 mRNA and protein levels were significantly reduced in the mouse hippocampus following anesthesia and surgery. ETV5 overexpression attenuated cognitive impairment, enhanced antioxidant capacity, and hampered caspase-3/GSDME-mediated pyroptosis, which was neutralized by AAV9-KIF23. Under Iso + LPS conditions, ETV5 overexpression enhanced HT22 neuronal viability and antioxidant defense, suppressed the cleavage of caspase-3 and GSDME, and diminished the release of IL-1β, IL-18, and LDH. Contrarily, its silencing had inverse effects on oxidative stress and pyroptosis, which was abrogated by KIF23 knockdown. Mechanistically, ETV5 directly bound to the sequence spanning from 1 to 700 bp upstream of the KIF23 gene transcription initiation site and repressed its transcription. Our findings suggest that ETV5/KIF23 may represent a promising therapeutic target for PND.
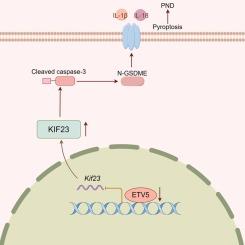
ETV5转录抑制KIF23抑制围手术期神经认知障碍老年小鼠焦亡。
Caspase-3/gasdermin E (GSDME)依赖性焦亡激活已成为围手术期神经认知障碍(PND)的潜在机制。公共转录组分析鉴定了老年PND小鼠海马中转录因子e - 26变体5 (ETV5)的减少。本研究旨在探讨ETV5及其靶基因在PND发病机制中的作用。生物信息学分析发现,kinesin家族成员23 (KIF23)可能是ETV5的靶基因。用重组腺相关病毒9 (AAV9)过表达ETV5和/或KIF23,经异氟醚麻醉下剖腹建立PND小鼠模型。HT22神经元分别用pcDNA3.1-ETV5或siETV5转染,然后用异氟烷和脂多糖(Iso + LPS)处理。评估认知行为、TUNEL染色和焦热相关指标。麻醉和手术后小鼠海马ETV5 mRNA和蛋白水平显著降低。ETV5过表达可减轻认知障碍,增强抗氧化能力,抑制caspase-3/ gsdme介导的焦亡,而这种焦亡可被AAV9-KIF23中和。在Iso + LPS条件下,ETV5过表达增强HT22神经元活力和抗氧化防御,抑制caspase-3和GSDME的裂解,减少IL-1β、IL-18和LDH的释放。相反,KIF23的沉默对氧化应激和焦亡有相反的作用,而这种作用被KIF23敲除而消除。机制上,ETV5直接结合KIF23基因转录起始位点上游1 ~ 700 bp的序列,抑制其转录。我们的研究结果表明,ETV5/KIF23可能是PND的一个有希望的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


