Hidden Plasmodium diversity revealed in southeastern Asian passerines using next-generation amplicon sequencing
IF 1.7
Q3 PARASITOLOGY
Current research in parasitology & vector-borne diseases
Pub Date : 2025-01-01
DOI:10.1016/j.crpvbd.2025.100319
引用次数: 0
Abstract
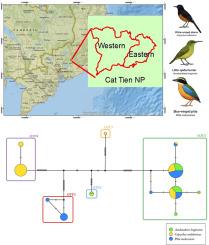
Monitoring haemosporidian (Genus Plasmodium) infections in passerine birds is essential for understanding the intricate dynamics of avian malaria and its implications for ecology and evolution of avian populations. In this study, we investigated the prevalence and diversity of malaria infections caused by Plasmodium species in three passerine species in Cat-Tien National Park, Vietnam. Using next-generation amplicon sequencing (NGS) of haemosporidian cytochrome b gene, we identified two known and ten novel Plasmodium lineages. Our genetic analysis revealed a high rate of Plasmodium infections in the little spiderhunter (Arachnothera longirostra; Nectariniidae), the white-rumped shama (Copsychus malabaricus; Muscicapidae) and the blue-winged pitta (Pitta moluccensis; Pittidae). Species delimitation methods identified five distinct operational taxonomic units (OTUs), consistently across the majority of the used methods. Each of the passerine species was infected with a specific subset of the total Plasmodium diversity. Phylogenetic analysis showed that sympatric Plasmodium OTUs are not closely related and possess overlapping host preferences. Our findings may reflect differences in habitat use, such as the vertical strata occupied by different bird species, which contribute to varying exposure levels to suitable vectors, thereby influencing infection rates and parasite diversity. Our findings corroborate the view that avian malaria parasites are not uniformly opportunistic; rather, their distribution is filtered by host identity and ecology. Understanding these dynamics is crucial for avian conservation and broader ecological studies, as avian malaria serves as a model for studying parasite-vector-host co-evolution and the impact of environmental changes on disease dynamics.
利用下一代扩增子测序揭示东南亚雀形目中隐藏的疟原虫多样性
监测雀形目鸟类中的血孢子虫(疟原虫属)感染对于了解禽疟疾的复杂动态及其对鸟类种群生态和进化的影响至关重要。本研究调查了越南猫天国家公园三种雀形目动物中疟原虫引起的疟疾感染的流行率和多样性。利用新一代血孢子虫细胞色素b基因扩增子测序(NGS),我们鉴定了两个已知的和十个新的疟原虫谱系。我们的遗传分析显示,小蜘蛛(Arachnothera longirostra; Nectariniidae)、白背沙马科(Copsychus malabaricus; Muscicapidae)和蓝翅皮塔(pitta moluccensis; Pittidae)的疟原虫感染率很高。物种划分方法确定了五个不同的操作分类单位(otu),在大多数使用的方法中是一致的。每一种雀形目动物都感染了总疟原虫多样性的一个特定子集。系统发育分析表明,同域疟原虫OTUs的亲缘关系并不密切,并且具有重叠的寄主偏好。我们的发现可能反映了栖息地利用的差异,例如不同鸟类所占据的垂直地层,这导致了不同的媒介暴露水平,从而影响了感染率和寄生虫多样性。我们的发现证实了禽疟寄生虫并非都是机会性的;相反,它们的分布是由宿主身份和生态过滤的。了解这些动态对鸟类保护和更广泛的生态学研究至关重要,因为禽疟可以作为研究寄生虫-媒介-宿主共同进化和环境变化对疾病动态影响的模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


