Exposure to low-dose CuO nanoparticles improves fear extinction memory and enhance intrinsic excitability in the infralimbic cortex of male mice
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Aims
Nanomaterials have gained considerable attention for diverse medical applications, particularly in neurology. Copper oxide nanoparticles (CuO-NPs) exhibit unique nanoscale properties that enable close interactions with neuronal cells, highlighting their potential as therapeutic agents for modulating synaptic plasticity and improving cognitive function. We aimed to investigate whether low-dose CuO-NPs can enhance fear extinction memory and increase intrinsic excitability in the infralimbic cortex (IL) of male mice.
Materials and methods
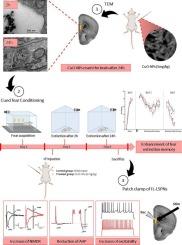
Adult male mice were subjected to fear conditioning using auditory cues paired with footshocks. Following the administration of a low dose of CuO-NPs, behavioral performance was assessed by measuring freezing responses during extinction. Whole-cell patch-clamp recordings were then performed on IL pyramidal neurons to evaluate intrinsic excitability and AMPA/NMDA ratio. Transmission electron microscopy (TEM) was applied after 2 and 24 h to assess the ability of CuO-NPs to reach the brain.
Key findings
We demonstrated via TEM that CuO-NPs efficiently cross the blood-brain barrier within 24 h' post-administration. Using cued fear conditioning, we subsequently found that a single intraperitoneal injection of CuO-NPs enhanced fear extinction memory, as evidenced by a significant reduction in freezing behavior compared to control animals. Patch-clamp analysis confirmed that CuO-NPs increased the excitability of IL pyramidal neurons and induced a sustained reduction in fast, medium, and slow post-spike after hyperpolarizations. Additionally, we demonstrated that CuO-NPs increased N-methyl-d-aspartate receptor-mediated synaptic currents, suggesting an enhanced synaptic plasticity in the infralimbic cortex.
Significance
Our findings offer new insights into the potential use of CuO-NPs for modulating fear extinction memory.
暴露于低剂量氧化铜纳米颗粒可改善雄性小鼠的恐惧消退记忆并增强边缘下皮层的内在兴奋性
纳米材料在各种医学应用中,特别是在神经病学方面,获得了相当大的关注。氧化铜纳米颗粒(CuO-NPs)表现出独特的纳米性质,能够与神经元细胞密切相互作用,突出了它们作为调节突触可塑性和改善认知功能的治疗剂的潜力。本实验旨在探讨低剂量CuO-NPs是否能增强雄性小鼠的恐惧消退记忆和增强边缘下皮层(IL)的内在兴奋性。材料与方法用听觉线索和脚震对成年雄性小鼠进行恐惧条件反射。在给予低剂量的CuO-NPs后,通过测量灭绝期间的冻结反应来评估行为表现。然后对IL锥体神经元进行全细胞膜片钳记录,以评估其固有兴奋性和AMPA/NMDA比值。在2 h和24 h后应用透射电镜(TEM)评估CuO-NPs到达大脑的能力。我们通过透射电镜证实,CuO-NPs在给药后24小时内有效地穿过血脑屏障。通过线索恐惧条件反射,我们随后发现单次腹腔注射CuO-NPs增强了恐惧消退记忆,与对照动物相比,冷冻行为显著减少。膜片钳分析证实,CuO-NPs增加了IL锥体神经元的兴奋性,并诱导超极化后快速、中速和慢速峰后的持续减少。此外,我们还证明了CuO-NPs增加了n -甲基-d-天冬氨酸受体介导的突触电流,表明边缘下皮层突触可塑性增强。我们的研究结果为CuO-NPs在调节恐惧消退记忆中的潜在应用提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


