Sigma-1 receptor counteracts non-cell-autonomous poly-PR-induced astrocytic oxidative stress in C9orf72 ALS
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
C9orf72-associated amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are characterized by the accumulation of toxic dipeptide repeat proteins (DPRs) generated from G4C2 hexanucleotide repeat expansions. Among these, the arginine-rich poly-PR (proline-arginine) species is the most neurotoxic, eliciting glial activation and neuroinflammation via non-cell-autonomous mechanisms. Although growing evidence implicates glial cells, particularly astrocytes, in disease progression, the molecular pathways linking neuron-derived poly-PR to astrocyte-mediated oxidative stress remain poorly understood. We demonstrate that exogenous poly-PR induces robust NOX4 expression and hydrogen peroxide (H2O2) production in astrocytes through activation of the IKK/IκB/NF-κB p65 signaling pathway. Mechanistically, poly-PR promotes nuclear translocation of p65 and enhances its binding to the NOX4 promoter, thereby amplifying astrocytic oxidative stress. Overexpression of the Sigma-1 receptor (Sigma-1R), an endoplasmic reticulum-resident chaperone, significantly attenuates poly-PR-induced NOX4 transcription and reactive oxygen species (ROS) production by interacting with p65 and blocking its nuclear translocation, independently of upstream p65 phosphorylation. Notably, clemastine, a clinically approved Sigma-1R agonist, suppresses astrocytic NOX4 expression by disrupting p65 binding to the NOX4 promoter. In a mouse model of C9orf72 ALS, Sigma-1R deficiency exacerbates poly-PR-induced neurodegeneration, astrogliosis, and NOX4 upregulation, whereas Sigma-1R sufficiency confers neuroprotection and anti-inflammatory effects. This study identifies Sigma-1R as a critical modulator of non-cell-autonomous poly-PR toxicity and establishes its activation as a potent suppressor of astrocyte-derived oxidative stress. Our findings uncover a previously unrecognized glial mechanism driving C9orf72 ALS pathogenesis and support Sigma-1R activation, via clemastine, as a promising therapeutic strategy to mitigate neuroinflammation and disease progression.
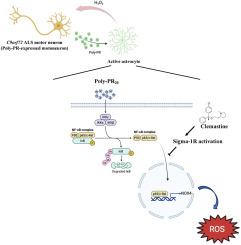
Sigma-1受体对抗非细胞自主多pr诱导的C9orf72 ALS星形细胞氧化应激
c9orf72相关的肌萎缩性侧索硬化症(ALS)和额颞叶痴呆(FTD)的特征是由G4C2六核苷酸重复扩增产生的有毒二肽重复蛋白(DPRs)积累。其中,富含精氨酸的多聚pr(脯氨酸-精氨酸)物种是最具神经毒性的,通过非细胞自主机制引发神经胶质激活和神经炎症。尽管越来越多的证据表明神经胶质细胞,特别是星形胶质细胞在疾病进展中起作用,但神经元衍生的多聚pr与星形胶质细胞介导的氧化应激之间的分子通路仍然知之甚少。我们证明,外源性多聚pr通过激活IKK/ i -κB /NF-κB p65信号通路,诱导星形胶质细胞中NOX4的表达和过氧化氢(H2O2)的产生。在机制上,poly-PR促进p65的核易位并增强其与NOX4启动子的结合,从而放大星形细胞氧化应激。Sigma-1受体(Sigma-1R)是一种内质网内伴侣,其过表达可通过与p65相互作用并阻断其核易位而显著减弱多pr诱导的NOX4转录和活性氧(ROS)的产生,而不依赖于上游p65磷酸化。值得注意的是,clemastine是一种临床批准的Sigma-1R激动剂,通过破坏p65与NOX4启动子的结合来抑制星形细胞NOX4的表达。在C9orf72 ALS小鼠模型中,Sigma-1R缺乏加剧了多pr诱导的神经变性、星形胶质细胞增生和NOX4上调,而Sigma-1R充足则具有神经保护和抗炎作用。本研究确定Sigma-1R是非细胞自主多pr毒性的关键调节剂,并确定其激活是星形胶质细胞衍生的氧化应激的有效抑制因子。我们的研究结果揭示了一种以前未被认识到的驱动C9orf72 ALS发病机制的神经胶质机制,并支持通过clemastine激活Sigma-1R作为一种有希望的治疗策略来减轻神经炎症和疾病进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


