Lead concentration derives phase alteration of hydroxylapatite
IF 3.4
3区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
Abstract
Hydroxylapatite is an important precipitant used in removing heavy metal contaminants through phosphate-induced metal stabilization (PIMS), where phase alteration plays a critical role. For lead pollution, the uptake mechanism is also affected by lead concentration. However, the mechanism in conjunction with lead concentration remains unclear, and the lead uptake mechanism remains different opinions. In this study, pathways of lead uptake and the influence of the initial lead concentration were systematically investigated using biogenic hydroxylapatite through the in-situ and ex-situ transmission electron microscope, XPS and Rietveld Refinement. The lead removal process was dominated by dissolution-precipitation and surface adsorption, without significant cation exchange. The findings indicate that concentration has a crucial effect on the lead uptake behavior of hydroxylapatite, especially for the precipitation of hydroxylpyromorphite. The heterogeneous growth of hydroxylpyromorphite gradually transformed into homogeneous growth with increasing lead concentration. Meanwhile, the total proportion of the dissolution-precipitation mechanism decreased with an increase in lead concentration, whereas the effect of the adsorption mechanism was likely enhanced by re-adsorption of lead by newly formed precipitates. The findings in this work provide a comprehensive insight into the mechanism of lead removal by hydroxylapatite, and infer that biogenic hydroxylapatite can play an important role in the environmental remediation of lead.
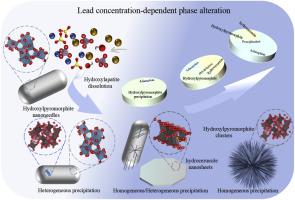
铅浓度来源于羟基磷灰石的相变
羟基磷灰石是一种重要的沉淀剂,用于通过磷酸盐诱导金属稳定(PIMS)去除重金属污染物,其中相变化起着关键作用。对于铅污染,其吸收机制也受铅浓度的影响。然而,与铅浓度有关的机制尚不清楚,铅的摄取机制仍有不同的看法。本研究利用生物源羟基磷灰石,通过原位和非原位透射电镜、XPS和Rietveld精密度分析,系统研究了铅的吸收途径和初始铅浓度的影响。铅的去除过程以溶解沉淀和表面吸附为主,没有明显的阳离子交换。研究结果表明,浓度对羟基磷灰石的铅吸收行为有重要影响,特别是对羟基焦闪石的沉淀。随着铅浓度的增加,羟基焦闪石的非均匀生长逐渐转变为均匀生长。同时,随着铅浓度的增加,溶解-沉淀机制的总比例降低,而新形成的沉淀物对铅的再吸附可能增强了吸附机制的效果。本研究结果全面揭示了羟基磷灰石除铅的机制,并推测生物源性羟基磷灰石在铅的环境修复中可能发挥重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Geochemistry
地学-地球化学与地球物理
CiteScore
6.10
自引率
8.80%
发文量
272
审稿时长
65 days
期刊介绍:
Applied Geochemistry is an international journal devoted to publication of original research papers, rapid research communications and selected review papers in geochemistry and urban geochemistry which have some practical application to an aspect of human endeavour, such as the preservation of the environment, health, waste disposal and the search for resources. Papers on applications of inorganic, organic and isotope geochemistry and geochemical processes are therefore welcome provided they meet the main criterion. Spatial and temporal monitoring case studies are only of interest to our international readership if they present new ideas of broad application.
Topics covered include: (1) Environmental geochemistry (including natural and anthropogenic aspects, and protection and remediation strategies); (2) Hydrogeochemistry (surface and groundwater); (3) Medical (urban) geochemistry; (4) The search for energy resources (in particular unconventional oil and gas or emerging metal resources); (5) Energy exploitation (in particular geothermal energy and CCS); (6) Upgrading of energy and mineral resources where there is a direct geochemical application; and (7) Waste disposal, including nuclear waste disposal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


