Methoxy-derivatized Pybox supports unique coordination structures in Ln(III) complexes
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Attempts at isolating complexes with a 2:1 ligand-to-metal ion stoichiometry for the ligand PyboxOMe led to a new Ce(III) complex with the expected stoichiometry, [Ce(PyboxOMe)2(NO3)3], where the metal ion has a coordination number of 12 in a distorted icosahedral geometry. However, in the case of other, smaller Ln(III) ions, four new complexes with a formal 3:2 ligand-to-metal stoichiometry were isolated, where one metal ion is surrounded by three PyboxOMe ligands, [Ln(PyboxOMe)3]3+ (Ln = Eu, Gd, Tb, Er), with a pseudo-D3 symmetry around the metal ion, a coordination number of 9 and a distorted tricapped trigonal prismatic geometry. A counter-anion is present, in which a second metal ion is bound to six bidentate nitrato anions, [Ln(NO3)6]3− for Ln = Eu, Gd, or to five bidentate nitrato anions in [Tb(NO3)5]2−, or to a mixture of bidentate and monodentate nitrato anions in [Er(NO3)6]2−. In addition to the slight decrease in Ln(III) ion size and ligand steric requirements, structural changes in these complexes along the Ln(III) series were attributed to crystal packing effects.
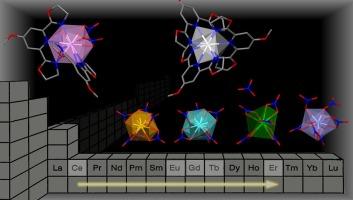
甲氧基衍生Pybox在Ln(III)配合物中支持独特的配位结构
尝试分离配体PyboxOMe与金属离子化学计量为2:1的配合物,得到了具有预期化学计量的新Ce(III)配合物[Ce(PyboxOMe)2(NO3)3],其中金属离子在扭曲的二十面体几何结构中具有12个配位数。然而,在其他较小的Ln(III)离子的情况下,分离出了四种新的配合物,其配体与金属的化学比为3:2,其中一个金属离子被三个PyboxOMe配体包围,[Ln(PyboxOMe)3]3+ (Ln = Eu, Gd, Tb, Er),金属离子周围具有伪d3对称,配位数为9,具有扭曲的三角棱柱几何形状。存在一个反阴离子,其中第二个金属离子与六个双齿硝酸盐阴离子结合,[Ln(NO3)6]3−对于Ln = Eu, Gd,或与[Tb(NO3)5]2−中的五个双齿硝酸盐阴离子结合,或与[Er(NO3)6]2−中的双齿和单齿硝酸盐阴离子的混合物结合。除了Ln(III)离子尺寸和配体空间要求的轻微降低外,这些配合物沿Ln(III)系列的结构变化可归因于晶体填充效应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


