Regulating the interaction mechanism of thiazole derivatives with mild steel through the conjugation effect: a combined DFT and MD simulation
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
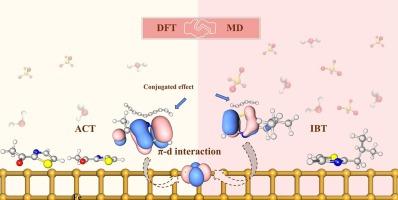
DFT calculations qualitatively and quantitatively reveal that increased charge accumulation at the 2-isobutylthiazole (IBT)/2-acetylthiazole (ACT) with Fe interface strengthens the bonding between heteroatom active sites and Fe atoms. ACT exhibits superior inhibition performance, with adsorption energies of −70.56 kcal/mol−1 for ACT compared to −61.33 kcal/mol−1 for IBT, attributed to its extended conjugation and the strongest Fe![]() O bonding energy (−1.08 eV). The interaction originates from π-d hybridization, where enhanced π/π* delocalization promotes orbital overlap with Fe d states, confirming that a parallel molecular orientation is most favorable. MD simulations under acidic conditions further demonstrate that both conjugation and active sites dictate the extent of π-d coupling. Greater surface coverage of ACT leads to a more compact and stable protective film that effectively blocks corrosive species. The results provide atomic level insights into the anticorrosion mechanism, which could help in understanding the organic-metal interface and designing more effective inhibitors.
O bonding energy (−1.08 eV). The interaction originates from π-d hybridization, where enhanced π/π* delocalization promotes orbital overlap with Fe d states, confirming that a parallel molecular orientation is most favorable. MD simulations under acidic conditions further demonstrate that both conjugation and active sites dictate the extent of π-d coupling. Greater surface coverage of ACT leads to a more compact and stable protective film that effectively blocks corrosive species. The results provide atomic level insights into the anticorrosion mechanism, which could help in understanding the organic-metal interface and designing more effective inhibitors.
通过共轭效应调节噻唑衍生物与低碳钢的相互作用机理:DFT和MD联合模拟
定性和定量的DFT计算表明,具有Fe界面的2-异丁基噻唑(IBT)/2-乙酰噻唑(ACT)的电荷积累增强了杂原子活性位点与Fe原子之间的键合。ACT的吸附能为- 70.56 kcal/mol−1,而IBT的吸附能为- 61.33 kcal/mol−1,这是由于ACT的扩展共轭和FeO键能最强(- 1.08 eV)。相互作用源于π-d杂化,其中π/π*离域的增强促进了轨道与Fe d态的重叠,证实了平行分子取向是最有利的。酸性条件下的动力学模拟进一步表明,共轭和活性位点决定了π-d耦合的程度。ACT更大的表面覆盖导致更紧凑和稳定的保护膜,有效地阻止腐蚀性物质。研究结果提供了原子水平上的腐蚀机理,有助于理解有机-金属界面和设计更有效的抑制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


