Extended CC bond chain controlled luminescence behavior of novel ESIPT-active benzothiazole-based fluorophore in near-infrared region: A TD-DFT insight
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
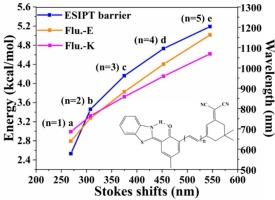
Using density functional theory (DFT) and time-dependent DFT (TD-DFT), the excited state intramolecular proton transfer (ESIPT) mechanisms and luminescent properties of (E)-2-(3-(3-(benzo[d]thiazol-2-yl)-2-hydroxy-5-methylstyryl)-5,5-dimethylcyclohex-2-en-1-ylidene) malononitrile (BTCMN) and its derivatives were systematically investigated. Four BTCMN derivatives with varying intramolecular C![]() C bond chain, namely BTCMN-1, BTCMN-2, BTCMN-3 and BTCMN-4 were designed to comprehensively explore the impact of C
C bond chain, namely BTCMN-1, BTCMN-2, BTCMN-3 and BTCMN-4 were designed to comprehensively explore the impact of C![]() C bond chain length on BTCMN. The selected functional is very reasonable since the experimental absorption and fluorescence wavelengths are reproduced. Detailed analyses have shown that the intramolecular C
C bond chain length on BTCMN. The selected functional is very reasonable since the experimental absorption and fluorescence wavelengths are reproduced. Detailed analyses have shown that the intramolecular C![]() C bond chain length obviously affects the ESIPT process and photophysical properties. With the elongation of the C
C bond chain length obviously affects the ESIPT process and photophysical properties. With the elongation of the C![]() C bond chain within the molecule, the excited state intramolecular hydrogen bond (IHB) weakens, the ESIPT barrier increases, and both absorption and fluorescence wavelengths shift red. Especially, the fluorescence wavelength achieves near-infrared region by extending the chain of intramolecular C
C bond chain within the molecule, the excited state intramolecular hydrogen bond (IHB) weakens, the ESIPT barrier increases, and both absorption and fluorescence wavelengths shift red. Especially, the fluorescence wavelength achieves near-infrared region by extending the chain of intramolecular C![]() C bond. Our results can provide more useful information to further design ESIPT-based fluorescent probes for living cell imaging.
C bond. Our results can provide more useful information to further design ESIPT-based fluorescent probes for living cell imaging.
扩展CC键链控制新型esipt活性苯并噻唑基荧光团在近红外区域的发光行为:TD-DFT洞察
利用密度泛函理论(DFT)和时间相关DFT (TD-DFT),系统研究了(E)-2-(3-(3-(苯并[d]噻唑-2-基)-2-羟基-5-甲基苯基)-5,5-二甲基环己基-2-烯-1-乙基)丙二腈(BTCMN)及其衍生物的激发态质子转移(ESIPT)机理和发光性质。设计4个分子内CC键链不同的BTCMN衍生物BTCMN-1、BTCMN-2、BTCMN-3和BTCMN-4,全面探讨CC键链长度对BTCMN的影响。所选择的功能是非常合理的,因为实验吸收和荧光波长的再现。详细分析表明,分子内CC键链长度对ESIPT过程和光物理性质有明显影响。随着分子内CC键链的延长,分子内激发态氢键(IHB)减弱,ESIPT势垒增加,吸收和荧光波长均红移。特别是,通过延长分子内CC键链,荧光波长达到近红外区域。我们的研究结果为进一步设计基于esipt的活细胞成像荧光探针提供了更多有用的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


